|
Water-reactive Substances
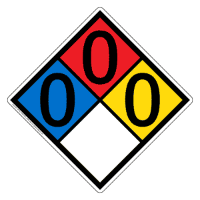
Water-reactive substances are those that spontaneously undergo a chemical reaction with water, as they are highly reducing in nature. Notable examples include alkali metals, lithium through caesium, and alkaline earth metals, magnesium through barium. Some water-reactive substances are also pyrophoric, like organometallics and sulphuric acid, and should be kept away from moisture. The use of acid-resistant gloves and face shield are required and should be handled in fume hoods. Such substances are classified as R2 under the UN classification system and as Hazard 4.3 by the United States Department of Transportation. In an NFPA 704 fire diamond's white square, they are denoted as "W̶". All chemicals that react vigorously with water or liberate toxic gas when in contact with water are recognized for their hazardous nature in the 'Approved Supply List,' or the list of substances covered by the international legislation on major hazards many of which are commonly used in manufactur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali Metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names for the elements in some languages, such as German and Russian. rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute Group (periodic table)#Group names, group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an atomic orbital, s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of periodic trends, group trends in properties in the periodic table, with elements exhibiting well-characterised homology (chemistry), homologous behaviour. This family of elements is also known as the lithium family after its leading element. The alkali metals are all sh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactivity (chemistry)
In chemistry, reactivity is the impulse for which a chemical substance undergoes a chemical reaction, either by itself or with other materials, with an overall release of energy. ''Reactivity'' refers to: * the chemical reactions of a single substance, * the chemical reactions of two or more substances that interact with each other, * the systematic study of sets of reactions of these two kinds, * methodology that applies to the study of reactivity of chemicals of all kinds, * experimental methods that are used to observe these processes * theories to predict and to account for these processes. The chemical reactivity of a single substance (reactant) covers its behavior in which it: * Decomposes * Forms new substances by addition of atoms from another reactant or reactants * Interacts with two or more other reactants to form two or more products The chemical reactivity of a substance can refer to the variety of circumstances (conditions that include temperature, pressure, prese ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Chemistry
Water chemistry analyses are carried out to identify and quantify the chemical components and properties of water samples. The type and sensitivity of the analysis depends on the purpose of the analysis and the anticipated use of the water. Chemical water analysis is carried out on water used in industrial processes, on waste-water stream, on rivers and stream, on rainfall and on the sea. In all cases the results of the analysis provides information that can be used to make decisions or to provide re-assurance that conditions are as expected. The analytical parameters selected are chosen to be appropriate for the decision making process or to establish acceptable normality. Water chemistry analysis is often the groundwork of studies of water quality, pollution, hydrology and geothermal waters. Analytical methods routinely used can detect and measure all the natural elements and their inorganic compounds and a very wide range of organic chemical species using methods such as gas chr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Safety
Chemicals as elements, compounds, mixtures, solutions and emulsions are very widely used and transported in the modern industrial society. Of necessity, they are also used in schools, universities and other training facilities to educate pupils in their safe use and handling and also are commonly used in domestic situations for cleaning, gardening and DIY. However, there are chemicals that should not mix or get in contact with others, as they can produce byproducts that may be toxic, carcinogenic, explosive etc, or can be dangerous themselves. To avoid disasters and mishaps, maintaining safety is considered paramount, especially by chemists. Chemical safety includes all those policies, procedures and practices designed to minimise the risk of exposure to potentially hazardous chemicals. This includes the risks of exposure to persons handling the chemicals, to the surrounding environment, and to the communities and ecosystems within that environment. The hazardous nature of many ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxides
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 (called a passivation layer) that protects the foil from further corrosion.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Stoichiometry (the measurable relationship between reactants and chemical equations of a equation or reaction) Oxides are extraordinarily diverse in terms of stoichiometries and in terms of the structures of each stoichiometry. Most elements form oxides of more than one stoichiometry. A well known example is carbon monoxide and carbon dioxide.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxides
Hydroxide is a polyatomic ion, diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually Self-ionization of water, minor constituent of water. It functions as a base (chemistry), base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salt (chemistry), salts, some of which dissociation (chemistry), dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemicals, commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalent bond, covalently bound functional group, group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry. Many inorganic substances which bear the word ''hydroxide'' in their names ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Basic (chemistry)
In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form Hydroxide ions OH−. These ions can react with hydrogen ions (H+ according to Arrhenius) from the dissociation of acids to form water in an acid–base reaction. A base was therefore a metal hydroxide such as NaOH or Ca(OH)2. Such aqueous hydroxide solutions were also described by certain characteristic properties. They are slippery to the touch, can taste bitter and change the color of pH indicators (e.g., turn red litmus paper blue). In water, by altering the autoionization equilibrium, bases yield solutions in which the hydrogen ion activity is lower than it is in pure water, i.e., the water has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Hydroxide
Metal hydroxides are hydroxides of metals. They are often strong bases. They consist of hydroxide anions and metallic cations. Some metal hydroxides, such as alkali metal hydroxides, ionize completely when dissolved. Certain metal hydroxides are weak electrolytes and dissolve only partially in aqueous solution. Examples * Aluminium hydroxide * Beryllium hydroxide * Cobalt(II) hydroxide * Copper(II) hydroxide * Curium hydroxide * Gold(III) hydroxide * Iron(II) hydroxide * Mercury(II) hydroxide * Nickel(II) hydroxide * Tin(II) hydroxide * Uranyl hydroxide * Zinc hydroxide * Zirconium(IV) hydroxide * Lithium hydroxide * Rubidium hydroxide * Cesium hydroxide * Sodium hydroxide * Potassium hydroxide Alkali metal hydroxides Other metal hydroxides * Gallium(III) hydroxide * Lead(II) hydroxide * Thallium(I) hydroxide * Thallium(III) hydroxide Role in soils In soils, it is assumed that larger amounts of natural phenols are released from decomposing plant litter ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Hydroxide
Magnesium hydroxide is the inorganic compound with the chemical formula Mg(OH)2. It occurs in nature as the mineral brucite. It is a white solid with low solubility in water (). Magnesium hydroxide is a common component of antacids, such as milk of magnesia. Preparation Treating the solution of different soluble magnesium salts with alkaline water induces the precipitation of the solid hydroxide Mg(OH)2: :Mg2+ + 2OH− → Mg(OH)2 As is the second most abundant cation present in seawater after , it can be economically extracted directly from seawater by alkalinisation as described here above. On an industrial scale, Mg(OH)2 is produced by treating seawater with lime (Ca(OH)2). A volume of (or 160,000 US gallons) of seawater gives about one ton of Mg(OH)2. Ca(OH)2 is far more soluble than Mg(OH)2 and drastically increases the pH value of seawater from 8.2 to 12.5. The less soluble precipitates because of the common ion effect due to the added by the dissolution of : : ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Vapor
(99.9839 °C) , - , Boiling point , , - , specific gas constant , 461.5 J/( kg·K) , - , Heat of vaporization , 2.27 MJ/kg , - , Heat capacity , 1.864 kJ/(kg·K) Water vapor, water vapour or aqueous vapor is the gaseous phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Water vapor is transparent, like most constituents of the atmosphere. Under typical atmospheric conditions, water vapor is continuously generated by evaporation and removed by condensation. It is less dense than most of the other constituents of air and triggers convection currents that can lead to clouds. Being a component of Earth's hydrosphere and hydrologic cycle, it is particularly abundant in Earth's atmosphere, where it acts as a greenhouse gas and warming feedback, contributing more to total greenhouse effect than non-condensable gases such as carbon dioxide an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 (called a passivation layer) that protects the foil from further corrosion.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Stoichiometry (the measurable relationship between reactants and chemical equations of a equation or reaction) Oxides are extraordinarily diverse in terms of stoichiometries and in terms of the structures of each stoichiometry. Most elements form oxides of more than one stoichiometry. A well known example is carbon monoxide and carbon dioxide.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)