|
Vincaminol
Vincaminol (C20H26N2O2) is a chemical that is a part of the Vinca alkaloid group, which were discovered in the 1950s by a Canadian scientist and are derived from '' Vinca minor'' (periwinkle). Vincaminol is not as well known as some of the other Vinca alkaloids such as vinblastine, vinorelbine, vincristine, and vindesine Vindesine, also termed Eldisine, is a semisynthetic vinca alkaloid derived from the flowering plant ''Catharanthus roseus.'' Like the natural (e.g. vinblastine and vincristine) and semisynthetic vinca alkaloids (e.g. vinorelbine and vinflunine) ..., which are the four main, medically useful Vinca alkaloids. Uses Vincaminol is used in to synthesize vincamine. References Vinca alkaloids Tryptamine alkaloids Quinolizidine alkaloids {{alkaloid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinca Alkaloid
''Vinca'' alkaloids are a set of anti-mitotic and anti-microtubule alkaloid agents originally derived from the periwinkle plant ''Catharanthus roseus'' (basionym ''Vinca rosea'') and other ''vinca'' plants. They block beta-tubulin polymerization in a dividing cell. Sources The Madagascan periwinkle ''Catharanthus roseus'' L. is the source for a number of important natural products, including catharanthine and vindoline and the vinca alkaloids it produces from them: leurosine and the chemotherapy agents vinblastine and vincristine, all of which can be obtained from the plant. The newer semi-synthetic chemotherapeutic agent vinorelbine is used in the treatment of non-small-cell lung cancer and is not known to occur naturally. However, it can be prepared either from vindoline and catharanthine or from leurosine, in both cases by synthesis of anhydrovinblastine, which "can be considered as the key intermediate for the synthesis of vinorelbine." The leurosine pathway uses the Nug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinca Minor
''Vinca minor'' (common names lesser periwinkle or dwarf periwinkle) is a species of flowering plant in the dogbane family, native to central and southern Europe, from Portugal and France north to the Netherlands and the Baltic States, east to the Caucasus, and also southwestern Asia in Turkey. Other vernacular names used in cultivation include small periwinkle, common periwinkle, and sometimes in the United States, myrtle or creeping myrtle. Description ''Vinca minor'' is a trailing subshrub, spreading along the ground and rooting along the stems to form large clonal colonies and occasionally scrambling up to high but never twining or climbing. The leaves are evergreen, opposite, long and broad, glossy dark green with a leathery texture and an entire margin. The flowers are solitary in the leaf axils and are produced mainly from early spring to mid summer but with a few flowers still produced into the autumn; they are violet-purple (pale purple or white in some cultivate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinca Alkaloids
''Vinca'' alkaloids are a set of anti-mitotic and anti-microtubule alkaloid agents originally derived from the periwinkle plant ''Catharanthus roseus'' (basionym ''Vinca rosea'') and other ''vinca'' plants. They block beta-tubulin polymerization in a dividing cell. Sources The Madagascan periwinkle ''Catharanthus roseus'' L. is the source for a number of important natural products, including catharanthine and vindoline and the vinca alkaloids it produces from them: leurosine and the chemotherapy agents vinblastine and vincristine, all of which can be obtained from the plant. The newer semi-synthetic chemotherapeutic agent vinorelbine is used in the treatment of non-small-cell lung cancer and is not known to occur naturally. However, it can be prepared either from vindoline and catharanthine or from leurosine, in both cases by synthesis of anhydrovinblastine, which "can be considered as the key intermediate for the synthesis of vinorelbine." The leurosine pathway uses the Nug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinblastine
Vinblastine (VBL), sold under the brand name Velban among others, is a chemotherapy medication, typically used with other medications, to treat a number of types of cancer. This includes Hodgkin's lymphoma, non-small cell lung cancer, bladder cancer, brain cancer, melanoma, and testicular cancer. It is given by injection into a vein. Most people experience some side effects. Commonly it causes a change in sensation, constipation, weakness, loss of appetite, and headaches. Severe side effects include low blood cell counts and shortness of breath. It should not be given to people who have a current bacterial infection. Use during pregnancy will likely harm the baby. Vinblastine works by blocking cell division. Vinblastine was isolated in 1958. An example of a natural herbal remedy that has since been developed into a conventional medicine, vinblastine was originally obtained from the Madagascar periwinkle. It is on the World Health Organization's List of Essential Medicines. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinorelbine
Vinorelbine (NVB), sold under the brand name Navelbine among others, is a chemotherapy medication used to treat a number of types of cancer. This includes breast cancer and non-small cell lung cancer. It is given by injection into a vein or by mouth. Common side effects include bone marrow suppression, pain at the site of injection, vomiting, feeling tired, numbness, and diarrhea. Other serious side effects include shortness of breath. Use during pregnancy may harm the baby. Vinorelbine is in the vinca alkaloid family of medications. It is believed to work by disrupting the normal function of microtubules and thereby stopping cell division. Vinorelbine was approved for medical use in the United States in 1994. It is on the World Health Organization's List of Essential Medicines. Medical uses Vinorelbine is approved for the treatment of non-small-cell lung cancer. It is used off-label for other cancers such as metastatic breast cancer. It is also active in rhabdomyosarcoma. Sid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vincristine
Vincristine, also known as leurocristine and marketed under the brand name Oncovin among others, is a chemotherapy medication used to treat a number of types of cancer. This includes acute lymphocytic leukemia, acute myeloid leukemia, Hodgkin's disease, neuroblastoma, and small cell lung cancer among others. It is given intravenously. Most people experience some side effects from vincristine treatment. Commonly it causes a change in sensation, hair loss, constipation, difficulty walking, and headaches. Serious side effects may include neuropathic pain, lung damage, or low white blood cells which increases the risk of infection. Use during pregnancy may result in birth defects. It works by stopping cells from dividing properly. It is vital that it not be given intrathecally, as this causes paralysis and in most cases, death. Vincristine was first isolated in 1961. It is on the World Health Organization's List of Essential Medicines. It is a vinca alkaloid that can be obtained ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vindesine
Vindesine, also termed Eldisine, is a semisynthetic vinca alkaloid derived from the flowering plant ''Catharanthus roseus.'' Like the natural (e.g. vinblastine and vincristine) and semisynthetic vinca alkaloids (e.g. vinorelbine and vinflunine) derived from this plant, vindesine is an inhibitor of mitosis that is used as a chemotherapy drug. By inhibiting mitosis, vinedsine blocks the proliferation of cells, particularly the rapidly proliferation cells of certain types of cancer. It is used, generally in combination with other chemotherapeutic drugs, in the treatment of various malignancies such as leukaemia, lymphoma, melanoma, breast cancer, and lung cancer Lung cancer, also known as lung carcinoma (since about 98–99% of all lung cancers are carcinomas), is a malignant lung tumor characterized by uncontrolled cell growth in tissues of the lung. Lung carcinomas derive from transformed, malign .... References Vinca alkaloids Mitotic inhibitors {{antineo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vincamine
Vincamine is a monoterpenoid indole alkaloid found in the leaves of ''Vinca minor'' (lesser periwinkle), comprising about 25-65% of its indole alkaloids by weight. It can also be synthesized from related alkaloids. Uses Vincamine is sold in Europe as a prescription medicine for the treatment of primary degenerative and vascular dementia. In the United States, it is permitted to be sold as a dietary supplement when labeled for use in adults for six months or less. Most common preparations are in the sustained release tablet forms. Chemistry Synthesis Tabersonine can be used for semi-synthesis of vincamine. Derivatives Vinpocetine is a synthetic derivative of vincamine used for cerebrovascular diseases and as dietary supplement. Vincamine derivatives have been also studied as anti addictive and antidiabetic agents. Research It may have nootropic effects. It has been investigated as novel anticancer drug. See also *Apparicine *Conophylline Conophylline is a autophagy induci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
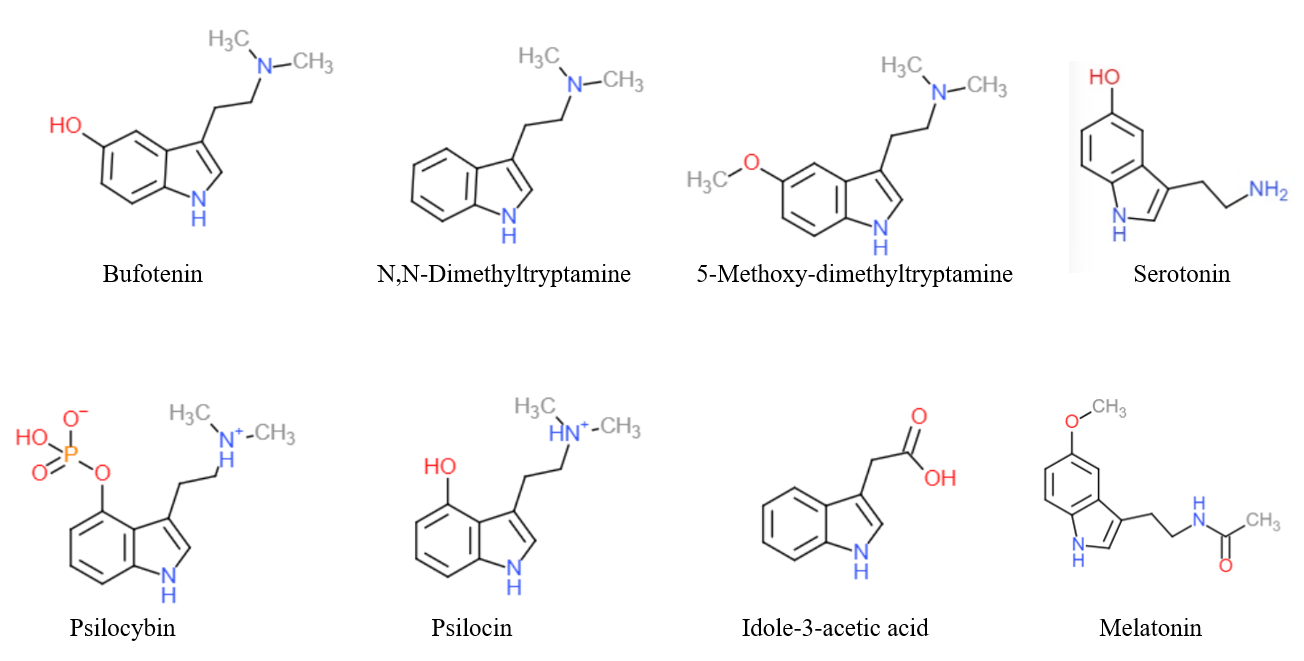
Tryptamine Alkaloids
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |