|
Vincamine
Vincamine is a monoterpenoid indole alkaloid found in the leaves of ''Vinca minor'' (lesser periwinkle), comprising about 25-65% of its indole alkaloids by weight. It can also be synthesized from related alkaloids. Uses Vincamine is sold in Europe as a prescription medicine for the treatment of primary degenerative and vascular dementia. In the United States, it is permitted to be sold as a dietary supplement when labeled for use in adults for six months or less. Most common preparations are in the sustained release tablet forms. Chemistry Synthesis Tabersonine can be used for semi-synthesis of vincamine. Derivatives Vinpocetine is a synthetic derivative of vincamine used for cerebrovascular diseases and as dietary supplement. Vincamine derivatives have been also studied as anti addictive and antidiabetic agents. Research It may have nootropic effects. It has been investigated as novel anticancer drug. See also *Apparicine *Conophylline Conophylline is a autophagy induci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinpocetine
Vinpocetine (ethyl apovincaminate) is a synthetic derivative of the vinca alkaloid vincamine, differing by the removal of a hydroxyl group. Vincamine is extracted from either the seeds of ''Voacanga africana'' or the leaves of ''Vinca minor'' (lesser periwinkle). Medical uses Vinpocetine has been used in many Asian and European countries for treatment of cerebrovascular disorders such as stroke and dementia for over three decades. Vinpocetine is not approved for any therapeutic use in the United States. The FDA has tentatively ruled that vinpocetine, due to its synthetic nature and proposed therapeutic uses, is ineligible to be marketed as dietary supplement under the Federal Food, Drug, and Cosmetic Act. Despite this, vinpocetine remains widely available in dietary supplements often marketed as nootropics. Vinpocetine is legally sold in CanadaRefer to the Health Canada website, for more details.Vinpocetine does not fully support a benefit in either dementia or stroke. As of 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indole Alkaloid
Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids. History The action of some indole alkaloids has been known for ages. Aztecs used the psilocybin mushrooms which contain alkaloids psilocybin and psilocin. The flowering plant ''Rauvolfia serpentina'' which contains reserpine was a common medicine in India around 1000 BC. Africans used the roots of the perennial rainforest shrub Iboga, which contain ibogaine, as a stimulant. An infusion of Calabar bean seeds was given to people accused of crime in Nigeria: its rejection by stomach was regarded as a sign of innoc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioorganic Chemistry
Bioorganic chemistry is a scientific discipline that combines organic chemistry and biochemistry. It is that branch of life science that deals with the study of biological processes using chemical methods. Protein and enzyme function are examples of these processes. Sometimes biochemistry is used interchangeably for bioorganic chemistry; the distinction being that bioorganic chemistry is organic chemistry that is focused on the biological aspects. While biochemistry aims at understanding biological processes using chemistry, bioorganic chemistry attempts to expand organic-chemical researches (that is, structures, synthesis, and kinetics) toward biology. When investigating metalloenzymes and cofactors, bioorganic chemistry overlaps bioinorganic chemistry. Sub disciplines Biophysical organic chemistry is a term used when attempting to describe intimate details of molecular recognition The term molecular recognition refers to the specific interaction between two or more molecules ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinolizidine Alkaloids
Quinolizidine alkaloids are natural products that have a quinolizidine structure; this includes the lupine alkaloids. Occurrence Quinolizidine alkaloids can be found in the plant family of legumes, especially in papilionaceous plants. While the lupine alkaloids (following their name) can be found in lupines, tinctorin, for example, was isolated from the dyer's broom. Examples More than 200 quinolizidine alkaloids are known which can be classified into 6 structural types: * the lupinine type with 34 known structures, including lupinine and its derivatives * the camoensine type with 6 known structures, including camoensin * the spartein type with 66 structures, including sparteine, lupanine, angustifoline * the α-pyridone type with 25 structures, including anagyrine and cytisine * the matrine type with 31 structures, including matrine * and the ormosanin type with 19 structures, including ormosanine. (–)-Lupinine Structural Formula V2.svg, (–)-lupinine (6R,7S,9S,1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Esters
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bounded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For example, protonation of methanol gives an electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
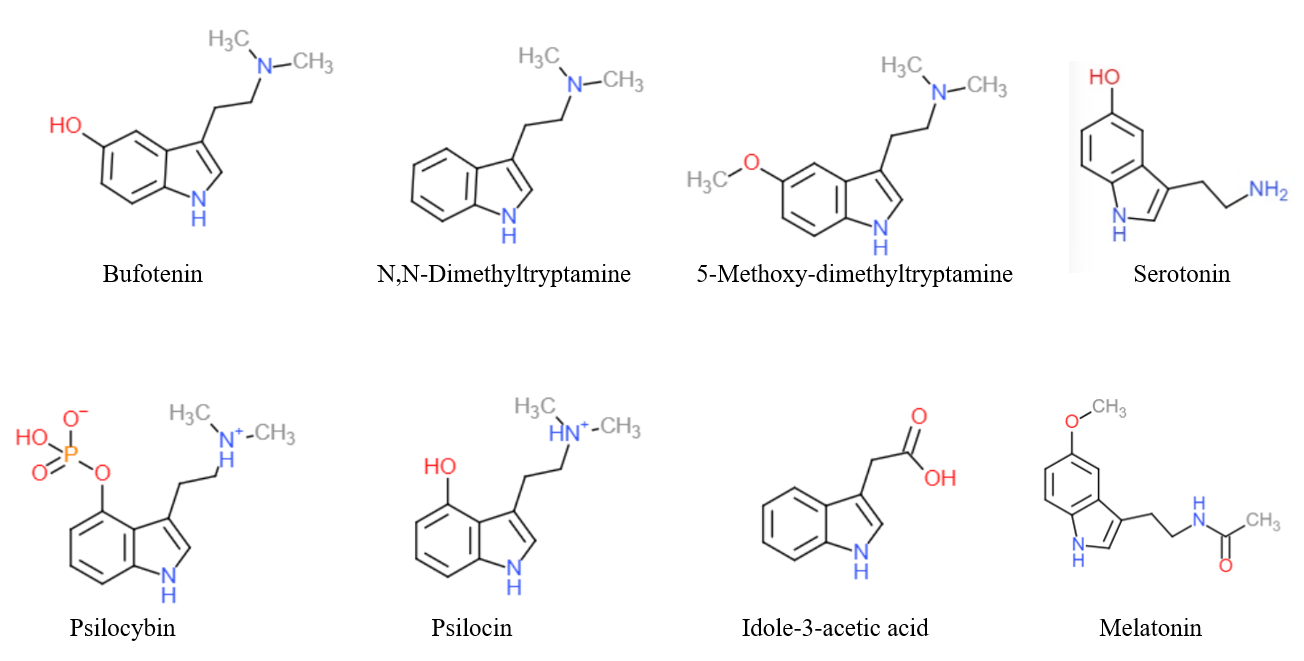
Tryptamine Alkaloids
Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the first one being the heterocyclic nitrogen). The structure of tryptamine is a shared feature of certain aminergic neuromodulators including melatonin, serotonin, bufotenin and psychedelic derivatives such as dimethyltryptamine (DMT), psilocybin, psilocin and others. Tryptamine has been shown to activate trace amine-associated receptors expressed in the mammalian brain, and regulates the activity of dopaminergic, serotonergic and glutamatergic systems. In the human gut, symbiotic bacteria convert dietary tryptophan to tryptamine, which activates 5-HT4 receptors and regulates gastrointestinal motility. Multiple tryptamine-derived drugs have been developed to treat migraines, while trace amine-associated receptors are being explored as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tertiary Alcohols
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conophylline
Conophylline is a autophagy inducing vinca alkaloid found in several species of ''Tabernaemontana'' including '' Ervatamia microphylla'' and ''Tabernaemontana divaricata''. Among its many functional groups is an epoxide: the compound where that ring is replaced with a double bond is called conophyllidine and this co-occurs in the same plants. History Conophylline and conophyllidine were first reported in 1993 after isolation from the ethanol extract of leaves of ''Tabernaemontana divaricata''. Their structures were confirmed by X-ray crystallography. The class of vinca alkaloids to which these compounds belong also contains vincristine and vinblastine, well-known therapeutic agents for human cancers, so they were candidates for a number of biochemical assays to see if they had useful biological activity. By 1996, conophylline it had been reported to inhibit tumours in rats by its action on Ras-expressing cells. This finding did not lead to a useful drug but the molecule continues t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apparicine
Apparicine is a monoterpenoid indole alkaloid. It is named after Apparicio Duarte, a Brazilian botanist who studied the ''Aspidosperma'' species from which apparicine was first isolated. It was the first member of the vallesamine group of alkaloids to be isolated and have its structure established, which was first published in 1965. It has also been known by the synonyms gomezine, pericalline, and tabernoschizine. Biochemistry The alkaloid has been isolated from seven species of ''Aspidosperma''. It is the principal alkaloid found in the callus of ''Tabernaemontana elegans'', and has also been identified in other ''Tabernaemontana'' species, including '' T. africana'', '' T. divaricata'', '' T. orientalis'', and '' T. pachysiphon''. In studies of ''T. pachysiphon'', it was found that alkaloid content including that of apparicine was greatest in young leaves and leaves receiving greater shade, and varied with leaf age, plant age, and provenance. Research on '' Aspidosperma pyri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nootropic
Nootropics ( , or ) (colloquial: smart drugs and cognitive enhancers, similar to adaptogens) are a wide range of natural or synthetic supplements or drugs and other substances that are claimed to improve cognitive function or to promote relaxation, particularly boosting mood, executive functions, attention, memory, creativity, or motivation in healthy individuals. The use of cognition-enhancing supplements by healthy individuals in the absence of a medical indication spans numerous controversial issues, including the ethics and fairness of their use, concerns over adverse effects, and the diversion of prescription drugs for non-medical uses. Nonetheless, the international sales of cognitive- or mood-enhancing supplements have continued to grow over time and in 2012 reached 0.69 billion. With sales supported by global health trends, the market is expected to reach US$33.85 billion by the year 2030, at a CAGR of 14.8%. While most nootropics are not regulated, there are ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinca Minor
''Vinca minor'' (common names lesser periwinkle or dwarf periwinkle) is a species of flowering plant in the dogbane family, native to central and southern Europe, from Portugal and France north to the Netherlands and the Baltic States, east to the Caucasus, and also southwestern Asia in Turkey. Other vernacular names used in cultivation include small periwinkle, common periwinkle, and sometimes in the United States, myrtle or creeping myrtle. Description ''Vinca minor'' is a trailing subshrub, spreading along the ground and rooting along the stems to form large clonal colonies and occasionally scrambling up to high but never twining or climbing. The leaves are evergreen, opposite, long and broad, glossy dark green with a leathery texture and an entire margin. The flowers are solitary in the leaf axils and are produced mainly from early spring to mid summer but with a few flowers still produced into the autumn; they are violet-purple (pale purple or white in some cultivate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Journal Of Medicinal Chemistry
The ''European Journal of Medicinal Chemistry'' is a monthly peer-reviewed scientific journal covering medicinal chemistry and published by Elsevier. It was established in 1966 as ''Chimica Therapeutica'' (CODEN: CHTPBA) and obtained its current title in 1974. From 1974 to 1981 the journal was still subtitled as ''Chimica Therapeutica'' and from 1982 to 1986 the subtitle was ''Chimie Thérapeutique'', indicating its French origin. And now it is the journal of the French Société de Chimie Thérapeutique. The journal covers research on all aspects of medicinal chemistry and publishes original papers, laboratory notes, short or preliminary communications, and invited reviews. The ''European Journal of Medicinal Chemistry'' is abstracted and indexed in the Index medicus and MEDLINE MEDLINE (Medical Literature Analysis and Retrieval System Online, or MEDLARS Online) is a bibliographic database of life sciences and biomedical information. It includes bibliographic information for a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |