|
Vibronic Coupling
Vibronic coupling (also called nonadiabatic coupling or derivative coupling) in a molecule involves the interaction between electronic and nuclear vibrational motion. The term "vibronic" originates from the combination of the terms "vibrational" and "electronic", denoting the idea that in a molecule, vibrational and electronic interactions are interrelated and influence each other. The magnitude of vibronic coupling reflects the degree of such interrelation. In theoretical chemistry, the vibronic coupling is neglected within the Born–Oppenheimer approximation. Vibronic couplings are crucial to the understanding of nonadiabatic processes, especially near points of conical intersections. The direct calculation of vibronic couplings used to be uncommon due to difficulties associated with its evaluation, but has recently gained popularity due to increased interest in the quantitative prediction of internal conversion rates, as well as the development of cheap but rigorous ways ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not consid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
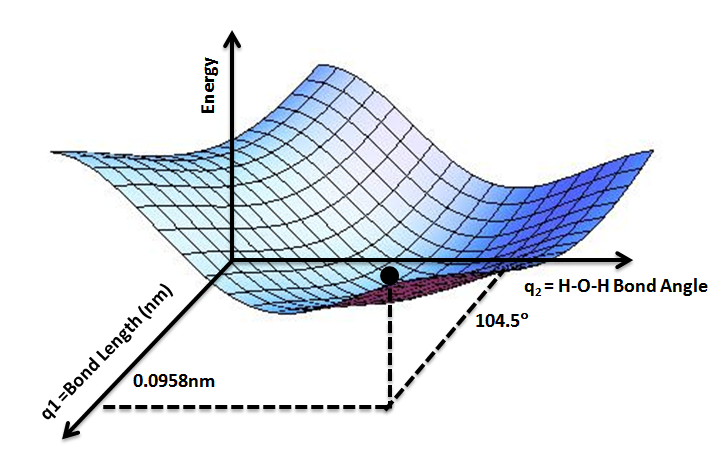
Potential Energy Surface
A potential energy surface (PES) describes the energy of a system, especially a collection of atoms, in terms of certain parameters, normally the positions of the atoms. The surface might define the energy as a function of one or more coordinates; if there is only one coordinate, the surface is called a ''potential energy curve'' or energy profile. An example is the Morse/Long-range potential. It is helpful to use the analogy of a landscape: for a system with two degrees of freedom (e.g. two bond lengths), the value of the energy (analogy: the height of the land) is a function of two bond lengths (analogy: the coordinates of the position on the ground). The PES concept finds application in fields such as chemistry and physics, especially in the theoretical sub-branches of these subjects. It can be used to theoretically explore properties of structures composed of atoms, for example, finding the minimum energy shape of a molecule or computing the rates of a chemical reaction. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maria Goeppert-Mayer
Maria Goeppert Mayer (; June 28, 1906 – February 20, 1972) was a German-born American theoretical physicist, and Nobel laureate in Physics for proposing the nuclear shell model of the atomic nucleus The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron i .... She was the second woman to win a Nobel Prize in physics, the first being Marie Curie. In 1986, the Maria Goeppert-Mayer Award for early-career women physicists was established in her honor. A graduate of the University of Göttingen, Goeppert Mayer wrote her doctoral thesis on the theory of possible two-photon absorption by atoms. At the time, the chances of experimentally verifying her thesis seemed remote, but the development of the laser in the 1960s later permitted this. Today, the unit for the two-photon absorption cross sect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structure, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. History Discovery The word "''benzene''" derives from "''gum benzoin''" (benzoin res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Excited State
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to an increase in energy level above a chosen starting point, usually the ground state, but sometimes an already excited state. The temperature of a group of particles is indicative of the level of excitation (with the notable exception of systems that exhibit negative temperature). The lifetime of a system in an excited state is usually short: spontaneous or induced emission of a quantum of energy (such as a photon or a phonon) usually occurs shortly after the system is promoted to the excited state, returning the system to a state with lower energy (a less excited state or the ground state). This return to a lower energy level is often loosely described as decay and is the inverse of excitation. Long-lived excited states are often called ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Empirical Method
Empirical research is research using empirical evidence. It is also a way of gaining knowledge by means of direct and indirect observation or experience. Empiricism values some research more than other kinds. Empirical evidence (the record of one's direct observations or experiences) can be analyzed quantitatively or qualitatively. Quantifying the evidence or making sense of it in qualitative form, a researcher can answer empirical questions, which should be clearly defined and answerable with the evidence collected (usually called data). Research design varies by field and by the question being investigated. Many researchers combine qualitative and quantitative forms of analysis to better answer questions that cannot be studied in laboratory settings, particularly in the social sciences and in education. In some fields, quantitative research may begin with a research question (e.g., "Does listening to vocal music during the learning of a word list have an effect on later memory ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MCSCF
Multi-configurational self-consistent field (MCSCF) is a method in quantum chemistry used to generate qualitatively correct reference states of molecules in cases where Hartree–Fock and density functional theory are not adequate (e.g., for molecular ground states which are quasi-degenerate with low-lying excited states or in bond-breaking situations). It uses a linear combination of configuration state functions (CSF), or configuration determinants, to approximate the exact electronic wavefunction of an atom or molecule. In an MCSCF calculation, the set of coefficients of both the CSFs or determinants and the basis functions in the molecular orbitals are varied to obtain the total electronic wavefunction with the lowest possible energy. This method can be considered a combination between configuration interaction (where the molecular orbitals are not varied but the expansion of the wave function) and Hartree–Fock (where there is only one determinant, but the molecular orbitals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Chemistry
Quantum chemistry, also called molecular quantum mechanics, is a branch of physical chemistry focused on the application of quantum mechanics to chemical systems, particularly towards the quantum-mechanical calculation of electronic contributions to physical and chemical properties of Molecule, molecules, Material, materials, and solutions at the atomic level. These calculations include systematically applied approximations intended to make calculations computationally feasible while still capturing as much information about important contributions to the computed Wave function, wave functions as well as to observable properties such as structures, spectra, and thermodynamic properties. Quantum chemistry is also concerned with the computation of quantum effects on molecular dynamics and chemical kinetics. Chemists rely heavily on spectroscopy through which information regarding the Quantization (physics), quantization of energy on a molecular scale can be obtained. Common metho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geometric Phase
In classical and quantum mechanics, geometric phase is a phase difference acquired over the course of a cycle, when a system is subjected to cyclic adiabatic processes, which results from the geometrical properties of the parameter space of the Hamiltonian. The phenomenon was independently discovered by S. Pancharatnam (1956), in classical optics and by H. C. Longuet-Higgins (1958)See page 12 in molecular physics; it was generalized by Sir Michael Berry in (1984). It is also known as the Pancharatnam–Berry phase, Pancharatnam phase, or Berry phase. It can be seen in the conical intersection of potential energy surfaces and in the Aharonov–Bohm effect. Geometric phase around the conical intersection involving the ground electronic state of the C6H3F3+ molecular ion is discussed on pages 385–386 of the textbook by Bunker and Jensen. In the case of the Aharonov–Bohm effect, the adiabatic parameter is the magnetic field enclosed by two interference paths, and it i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Path Integral Molecular Dynamics
Path integral molecular dynamics (PIMD) is a method of incorporating quantum mechanics into molecular dynamics simulations using Feynman path integrals. In PIMD, one uses the Born–Oppenheimer approximation to separate the wavefunction into a nuclear part and an electronic part. The nuclei are treated quantum mechanically by mapping each quantum nucleus onto a classical system of several fictitious particles connected by springs (harmonic potentials) governed by an effective Hamiltonian, which is derived from Feynman's path integral. The resulting classical system, although complex, can be solved relatively quickly. There are now a number of commonly used condensed matter computer simulation techniques that make use of the path integral formulation including Centroid Molecular Dynamics (CMD), Ring Polymer Molecular Dynamics (RPMD), and the Feynman-Kleinert Quasi-Classical Wigner (FK-QCW) method. The same techniques are also used in path integral Monte Carlo Path integral Monte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Hopping
Surface hopping is a mixed quantum-classical technique that incorporates quantum mechanical effects into molecular dynamics simulations. Traditional molecular dynamics assume the Born-Oppenheimer approximation, where the lighter electrons adjust instantaneously to the motion of the nuclei. Though the Born-Oppenheimer approximation is applicable to a wide range of problems, there are several applications, such as photoexcited dynamics, electron transfer, and surface chemistry where this approximation falls apart. Surface hopping partially incorporates the non-adiabatic effects by including excited adiabatic surfaces in the calculations, and allowing for 'hops' between these surfaces, subject to certain criteria. Motivation Molecular dynamics simulations numerically solve the classical equations of motion. These simulations, though, assume that the forces on the electrons are derived solely by the ground adiabatic surface. Solving the time-dependent Schrödinger equation numeri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
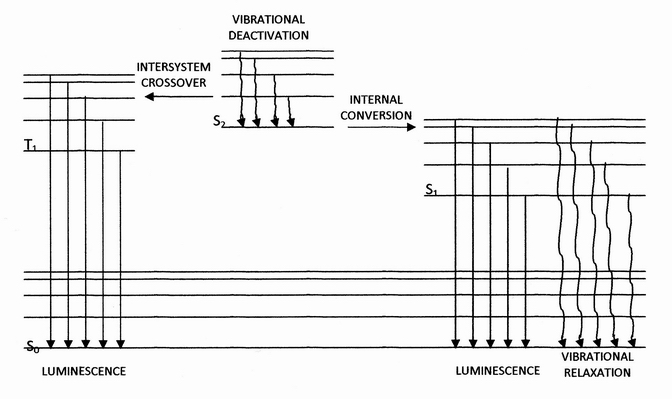
Internal Conversion (chemistry)
Internal conversion is a transition from a higher to a lower electronic state in a molecule or atom.A general and quantitative discussion of intramolecular radiationless transitions is the subject of an article by M. Bixon and J. Jortner (''J. Chem. Phys.'', 48 (2) 715-726 (1968)). It is sometimes called "radiationless de-excitation", because no photons are emitted. It differs from intersystem crossing in that, while both are radiationless methods of de-excitation, the molecular spin state for internal conversion remains the same, whereas it changes for intersystem crossing. The energy of the electronically excited state is given off to vibrational modes of the molecule. The excitation energy is transformed into heat. Examples A classic example of this process is the quinine sulfate fluorescence, which can be quenched by the use of various halide salts. The excited molecule can de-excite by increasing the thermal energy of the surrounding solvated ions. Several natural molecules ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


2.png)