|
Tyramine
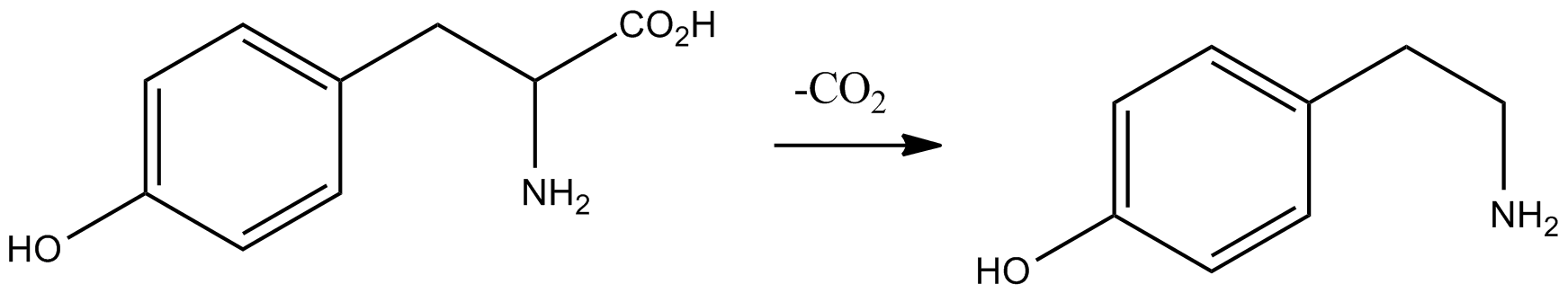
Tyramine ( ) (also spelled tyramin), also known under several other names, is a naturally occurring trace amine derived from the amino acid tyrosine. Tyramine acts as a catecholamine releasing agent. Notably, it is unable to cross the blood-brain barrier, resulting in only non-psychoactive peripheral sympathomimetic effects following ingestion. A hypertensive crisis can result, however, from ingestion of tyramine-rich foods in conjunction with the use of monoamine oxidase inhibitors (MAOIs). Occurrence Tyramine occurs widely in plants and animals, and is metabolized by various enzymes, including monoamine oxidases. In foods, it often is produced by the decarboxylation of tyrosine during fermentation or decay. Foods that are fermented, cured, pickled, aged, or spoiled have high amounts of tyramine. Tyramine levels go up when foods are at room temperature or go past their freshness date. Specific foods containing considerable amounts of tyramine include: * strong or a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine Beta-hydroxylase
Dopamine beta-hydroxylase (DBH), also known as dopamine beta-monooxygenase, is an enzyme () that in humans is encoded by the DBH gene. Dopamine beta-hydroxylase catalyzes the conversion of dopamine to norepinephrine. The three substrates of the enzyme are dopamine, vitamin C (ascorbate), and O2. The products are norepinephrine, dehydroascorbate, and H2O. DBH is a 290 kDa copper-containing oxygenase consisting of four identical subunits, and its activity requires ascorbate as a cofactor. It is the only enzyme involved in the synthesis of small-molecule neurotransmitters that is membrane-bound, making norepinephrine the only known transmitter synthesized inside vesicles. It is expressed in noradrenergic neurons of the central nervous system (i.e. locus coeruleus) and peripheral nervous systems (i.e. sympathetic ganglia), as well as in chromaffin cells of the adrenal medulla. Mechanism of catalysis Based on the observations of what happens when there is no substrate, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trace Amine
Trace amines are an endogenous group of trace amine-associated receptor 1 (TAAR1) agonists – and hence, monoaminergic neuromodulators – that are structurally and metabolically related to classical monoamine neurotransmitters. Compared to the classical monoamines, they are present in trace concentrations. They are distributed heterogeneously throughout the mammalian brain and peripheral nervous tissues and exhibit high rates of metabolism. Although they can be synthesized within parent monoamine neurotransmitter systems, there is evidence that suggests that some of them may comprise their own independent neurotransmitter systems. Trace amines play significant roles in regulating the quantity of monoamine neurotransmitters in the synaptic cleft of monoamine neurons with . They have well-characterized presynaptic ''amphetamine-like'' effects on these monoamine neurons via TAAR1 activation; specifically, by activating TAAR1 in neurons they promote the release and prevent reup ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octopamine
Octopamine (molecular formula C8H11NO2; also known as OA, and also norsynephrine, ''para''-octopamine and others) is an organic chemical closely related to norepinephrine, and synthesized biologically by a homologous pathway. Octopamine is often considered the major "fight-or-flight" neurohormone of invertebrates. Its name is derived from the fact that it was first identified in the salivary glands of the octopus. In many types of invertebrates octopamine is an important neurotransmitter and hormone. In protostomes — arthropods, molluscs, and several types of worms — it substitutes for norephinephrine and performs functions apparently similar to those of norepinephrine in mammals, functions that have been described as mobilizing the body and nervous system for action. In mammals octopamine is found only in trace amounts, and no biological function has been solidly established for it. It is also found naturally in numerous plants, including bitter orange. Octopamine has bee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic chemical of the catecholamine and phenethylamine families. Dopamine constitutes about 80% of the catecholamine content in the brain. It is an amine synthesized by removing a carboxyl group from a molecule of its precursor chemical, L-DOPA, which is synthesized in the brain and kidneys. Dopamine is also synthesized in plants and most animals. In the brain, dopamine functions as a neurotransmitter—a chemical released by neurons (nerve cells) to send signals to other nerve cells. Neurotransmitters are synthesized in specific regions of the brain, but affect many regions systemically. The brain includes several distinct dopamine pathways, one of which plays a major role in the motivational component of reward-motivated behavior. The anticipation of most types of rewards increases the level of dopamine in the brain, and ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Oxidase Inhibitor
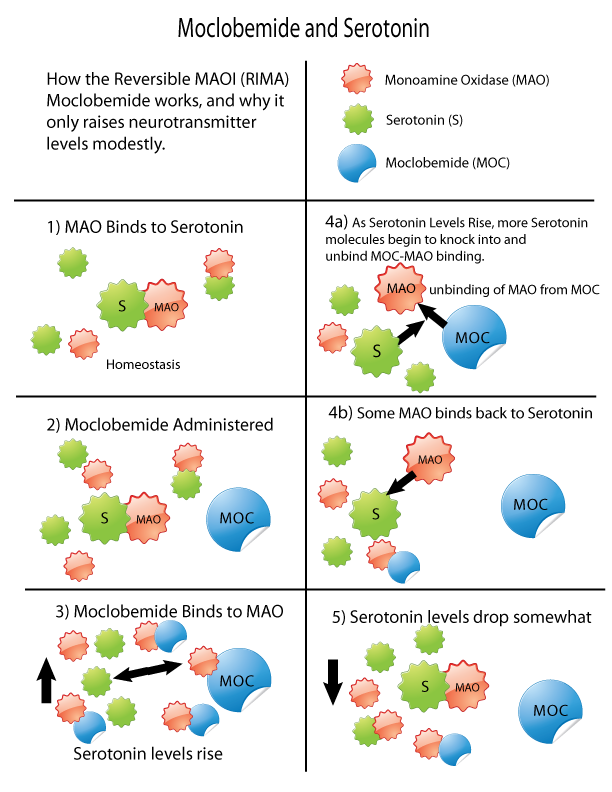
Monoamine oxidase inhibitors (MAOIs) are a class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especially for treatment-resistant depression and atypical depression. They are also used to treat panic disorder, social anxiety disorder, Parkinson's disease, and several other disorders. Reversible inhibitors of monoamine oxidase A (RIMAs) are a subclass of MAOIs that selectively and reversibly inhibit the MAO-A enzyme. RIMAs are used clinically in the treatment of depression and dysthymia. Due to their reversibility, they are safer in single-drug overdose than the older, irreversible MAOIs, and weaker in increasing the monoamines important in depressive disorder. RIMAs have not gained widespread market share in the United States. Medical uses MAOIs have been found to be effective in the treatment of panic disorder with ago ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek ''tyrós'', meaning ''cheese'', as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is encoded by the codons UAC and UAU in messenger RNA. Functions Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of the phenol functionality. It occurs in proteins that are part of signal transduction processes and functions as a receiver of phosphate groups that are transferred by way of protein kinases. Phosphorylation of the hydroxyl group can change the activity of the targ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Oxidase
Monoamine oxidases (MAO) () are a family of enzymes that catalyze the oxidation of monoamines, employing oxygen to clip off their amine group. They are found bound to the outer membrane of mitochondria in most cell types of the body. The first such enzyme was discovered in 1928 by Mary Bernheim in the liver and was named tyramine oxidase. The MAOs belong to the protein family of flavin-containing amine oxidoreductases. MAOs are important in the breakdown of monoamines ingested in food, and also serve to inactivate monoamine neurotransmitters. Because of the latter, they are involved in a number of psychiatric and neurological diseases, some of which can be treated with monoamine oxidase inhibitors (MAOIs) which block the action of MAOs. Subtypes and tissue distribution In humans there are two types of MAO: MAO-A and MAO-B. * Both are found in neurons and astroglia. * Outside the central nervous system: ** MAO-A is also found in the liver, pulmonary vascular endot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavin-containing Monooxygenase 3
Flavin-containing monooxygenase 3 (FMO3), also known as dimethylaniline monooxygenase -oxide-forming3 and trimethylamine monooxygenase, is a flavoprotein enzyme () that in humans is encoded by the ''FMO3'' gene. This enzyme catalyzes the following chemical reaction, among others: :trimethylamine + NADPH + H+ + O2 \rightleftharpoons trimethylamine ''N''-oxide + NADP+ + H2O FMO3 is the main flavin-containing monooxygenase isoenzyme that is expressed in the liver of adult humans. The human FMO3 enzyme catalyzes several types of reactions, including: the of primary, secondary, and tertiary amines; the of nucleophilic sulfur-containing compounds; and the of the anti-cancer agent dimethylxanthenone acetic acid (DMXAA). FMO3 is the primary enzyme in humans which catalyzes the ''N''-oxidation of trimethylamine into trimethylamine ''N''-oxide; FMO1 also does this, but to a much lesser extent than FMO3. Genetic deficiencies of the FMO3 enzyme cause primary trimethylaminuria, also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Releasing Agent
A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of a monoamine neurotransmitter from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitter. Many drugs induce their effects in the body and/or brain via the release of monoamine neurotransmitters, e.g., trace amines, many substituted amphetamines, and related compounds. Types of MRAs MRAS can be classified by the monoamines they mainly release, although these drugs lie on a spectrum. * Selective for one neurotransmitter ** Serotonin releasing agent (SRA) ** Norepinephrine releasing agent (NRA) ** Dopamine releasing agent (DRA) * Non-selective, releasing two or more neurotransmitters ** Norepinephrine–dopamine releasing agent (NDRA) ** Serotonin–norepinephrine releasing agent (SNRA) ** Serotonin–dopamine releasing agent (SDRA) ** Serotonin–norepinephrine–dopamine releasing agent (SNDRA) Mechani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-Methyltyramine
''N''-Methyltyramine (NMT), also known as 4-hydroxy-''N''-methylphenethylamine, is a human trace amine and natural phenethylamine alkaloid found in a variety of plants.T. A. Smith (1977). "Phenethylamine and related compounds in plants." ''Phytochemistry'' 16 9 – 18. As the name implies, it is the N-methyl analog of tyramine, which is a well-known biogenic trace amine with which NMT shares many pharmacological properties. Biosynthetically, NMT is produced by the N-methylation of tyramine via the action of the enzyme phenylethanolamine ''N''-methyltransferase in humans and tyramine ''N''-methyltransferase in plants. Occurrence N-methyltyramine seems to be quite widely distributed in plants. NMT was isolated as a natural product for the first time, from germinating barley roots, by Kirkwood and Marion in 1950. These chemists found that 600 g of barley, after germination and 10-day growth, yielded 168 mg of N-methyltyramine.S. Kirkwood and L. Marion (1950) ''J. Am. Chem. S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood–brain Barrier
The blood–brain barrier (BBB) is a highly selective semipermeable border of endothelial cells that prevents solutes in the circulating blood from ''non-selectively'' crossing into the extracellular fluid of the central nervous system where neurons reside. The blood–brain barrier is formed by endothelial cells of the capillary wall, astrocyte end-feet ensheathing the capillary, and pericytes embedded in the capillary basement membrane. This system allows the passage of some small molecules by passive diffusion, as well as the selective and active transport of various nutrients, ions, organic anions, and macromolecules such as glucose and amino acids that are crucial to neural function. The blood–brain barrier restricts the passage of pathogens, the diffusion of solutes in the blood, and large or hydrophilic molecules into the cerebrospinal fluid, while allowing the diffusion of hydrophobic molecules (O2, CO2, hormones) and small non-polar molecules. Cells of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peripheral Nervous System
The peripheral nervous system (PNS) is one of two components that make up the nervous system of bilateral animals, with the other part being the central nervous system (CNS). The PNS consists of nerves and ganglia, which lie outside the brain and the spinal cord. The main function of the PNS is to connect the CNS to the limbs and organs, essentially serving as a relay between the brain and spinal cord and the rest of the body. Unlike the CNS, the PNS is not protected by the vertebral column and skull, or by the blood–brain barrier, which leaves it exposed to toxins. The peripheral nervous system can be divided into the somatic nervous system and the autonomic nervous system. In the somatic nervous system, the cranial nerves are part of the PNS with the exception of the optic nerve (cranial nerve II), along with the retina. The second cranial nerve is not a true peripheral nerve but a tract of the diencephalon. Cranial nerve ganglia, as with all ganglia, are part of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |