|
Trimethylsilyl Trifluoromethanesulfonate
Trimethylsilyl trifluoromethanesulfonate is a trifluoromethanesulfonate derivate with a trimethylsilyl R-group. It has similar reactivity to trimethylsilyl chloride, and is also used often in organic synthesis. Illustrative reactions A common application is the conversion of ketones and aldehydes to silyl enol ethers. The stereoselective synthesis of seven benzylated proanthocyanidin trimers (epicatechin-(4β-8)-epicatechin-(4β-8)-epicatechin trimer (procyanidin C1), catechin-(4α-8)-catechin-(4α-8)-catechin trimer (procyanidin C2), epicatechin-(4β-8)-epicatechin-(4β-8)-catechin trimer and epicatechin-(4β-8)-catechin-(4α-8)-epicatechin trimer derivatives) can be achieved with TMSOTf-catalyzed condensation reaction, in excellent yields. Deprotection of (+)-catechin and (−)-epicatechin trimers derivatives gives four natural procyanidin trimers in good yields. It has been used in Takahashi Taxol total synthesis or for chemical glycosylation reactions. Trimethylsilyl triflu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoromethanesulfonate
In organic chemistry, triflate (Preferred IUPAC name, systematic name: trifluoromethanesulfonate), is a functional group with the Chemical formula, formula and Chemical structure, structure . The triflate group is often represented by , as opposed to −Tf, which is the trifluoromethylsulfonyl, triflyl group, . For example, Butyl group, ''n''-butyl triflate can be written as . The corresponding triflate Ion, anion, , is an extremely stable polyatomic ion; this comes from the fact that triflic acid () is a superacid; i.e. it is more acidic than pure sulfuric acid, already one of the strongest acids known. Applications A triflate group is an excellent leaving group used in certain organic reactions such as nucleophilic substitution, Suzuki couplings and Heck reactions. Since alkyl triflates are extremely reactive in SN2 reaction, SN2 reactions, they must be stored in conditions free of nucleophiles (such as water). The anion owes its stability to resonance stabilization which cau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylsilyl
A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom minus;Si(CH3)3 which is in turn bonded to the rest of a molecule. This structural group is characterized by chemical inertness and a large molecular volume, which makes it useful in a number of applications. A trimethylsilyl group bonded to a methyl group forms tetramethylsilane, which is abbreviated as TMS as well. Compounds with trimethylsilyl groups are not normally found in nature. Chemists sometimes use a trimethylsilylating reagent to derivatize rather non-volatile compounds such as certain alcohols, phenols, or carboxylic acids by substituting a trimethylsilyl group for a hydrogen in the hydroxyl groups on the compounds. This way trimethylsiloxy groups minus;O-Si(CH3)3are formed on the molecule. A couple of examples of trimethylsilylating agents include trimethylsilyl chloride and bis(trimethylsilyl)acetamide. Trime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylsilyl Chloride
Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound ( silyl halide), with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry. Preparation TMSCl is prepared on a large scale by the '' direct process'', the reaction of methyl chloride with a silicon-copper alloy. The principal target of this process is dimethyldichlorosilane, but substantial amounts of the trimethyl and monomethyl products are also obtained. The relevant reactions are (Me = CH3): : x MeCl + Si → Me3SiCl, Me2SiCl2, MeSiCl3, other products Typically about 2–4% of the product stream is the monochloride, which forms an azeotrope with MeSiCl3. Reactions and uses TMSCl is reactive toward nucleophiles, resulting in the replacement of the chloride. In a characteristic reaction of TMSCl, the nucleophile is water, resulting in hydrolysis to give the hexamethy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Procyanidin C1
Procyanidin C1 (PCC1) is a B type proanthocyanidin. It is an epicatechin trimer found in grape (''Vitis vinifera''), unripe apples, and cinnamon. Natural occurrence and function Procyanidin C1 can be isolated from grape seed extract. Chemical synthesis The stereoselective synthesis of seven benzylated proanthocyanidin trimers (epicatechin-(4β-8)-epicatechin-(4β-8)-epicatechin trimer (procyanidin C1), catechin-(4α-8)-catechin-(4α-8)-catechin trimer (procyanidin C2), epicatechin-(4β-8)-epicatechin-(4β-8)-catechin trimer and epicatechin-(4β-8)-catechin-(4α-8)-epicatechin trimer derivatives) can be achieved with Trimethylsilyl trifluoromethanesulfonate, TMSOTf-catalyzed condensation reaction, in excellent yields. The structure of benzylated procyanidin C2 was confirmed by comparing the 1H NMR spectra of protected procyanidin C2 that was synthesized by two different condensation approaches. Finally, deprotection of (+)-catechin and (-)-epicatechin trimers derivatives gives ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Procyanidin C2
Procyanidin C2 is a B type proanthocyanidin trimer, a type of condensed tannin. Natural occurrences Procyanidin C2 is found in grape seeds (''Vitis vinifera'') and wine, in barley (''Hordeum vulgare''), malt and beer, in ''Betula spp.'', in ''Pinus radiata'', in '' Potentilla viscosa'', in ''Salix caprea'' or in ''Cryptomeria japonica''. The contents in barley grain of trimeric proanthocyanidins, including procyanidin C2, range from 53 to 151 μg catechin equivalents/g. Possible health uses Proanthocyanidin oligomers, extracted from grape seeds, have been used for the experimental treatment of androgenic alopecia. When applied topically, they promote hair growth ''in vitro'', and induce anagen ''in vivo''. Procyanidin C2 is the subtype of extract most effective. Experiments showed that both procyanidin C2 and Pycnogenol (French maritime pine bark extract) increase TNF-α secretion in a concentration- and time-dependent manner. These results demonstrate that procyanidins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Takahashi Taxol Total Synthesis
The Takahashi Taxol total synthesis published by Takashi Takahashi in 2006 is one of several methods in taxol total synthesis.''A Formal Total Synthesis of Taxol Aided by an Automated Synthesizer'' Takayuki Doi, Shinichiro Fuse, Shigeru Miyamoto, Kazuoki Nakai, Daisuke Sasuga and Takashi Takahashi Chemistry an Asian J.; (Article); 2006; 1(3); 370-383. DOAbstract/ref> The method starts from geraniol and differs from the other 6 published methods that it is a formal synthesis (the final product is baccatin III which lacks the amide tail found in taxol itself) and that it is racemic (the product baccatin III is optically inactive). A key feature of the published procedure is that several synthetic steps (construction of rings A, B and C) were performed in an automated synthesizer on a scale up to 300 gram and that purification steps were also automated. A ring synthesis Ring A was synthesised starting from geraniol 1 and involved acylation ( acetic anhydride, DMAP, Et3N) to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Glycosylation
A chemical glycosylation reaction involves the coupling of a glycosyl donor, to a glycosyl acceptor forming a glycoside. If both the donor and acceptor are sugars, then the product is an oligosaccharide. The reaction requires activation with a suitable activating reagent. The reactions often result in a mixture of products due to the creation of a new stereogenic centre at the anomeric position of the glycosyl donor. The formation of a glycosidic linkage allows for the synthesis of complex polysaccharides which may play important roles in biological processes and pathogenesis and therefore having synthetic analogs of these molecules allows for further studies with respect to their biological importance. Terminology The glycosylation reaction involves the coupling of a glycosyl donor and a glycosyl acceptor via initiation using an activator under suitable reaction conditions. * A glycosyl donor is a sugar with a suitable leaving group at the anomeric position. This group, u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
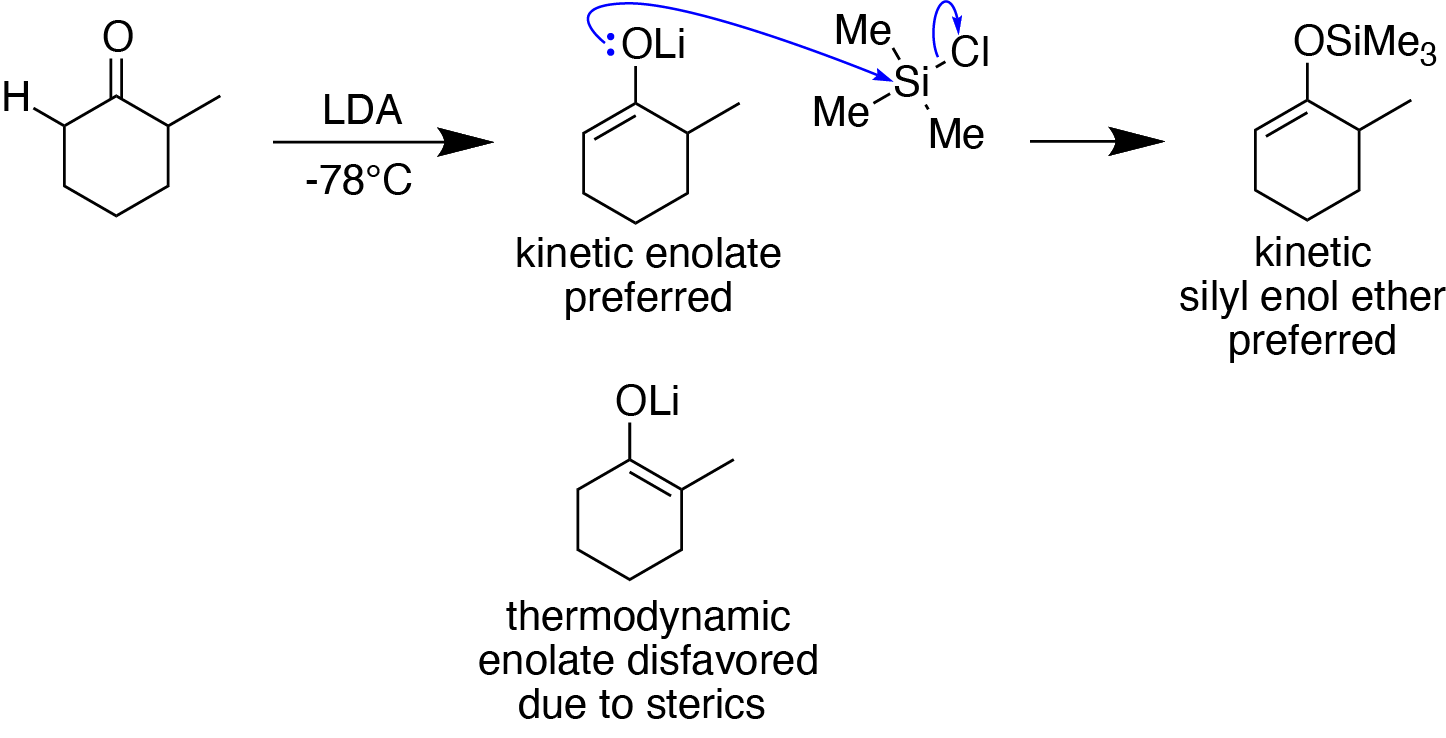
Silyl Enol Ether
Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen end to an organosilicon group. They are important intermediates in organic synthesis. Synthesis Silyl enol ethers are generally prepared by reacting an enolizable Carbonyl group, carbonyl compound with a silyl electrophile and a Base (chemistry), base, or just reacting an enolate with a silyl electrophile.Clayden, J., Greeves, N., & Warren, S. (2012). Silyl enol ethers. In ''Organic chemistry'' (Second ed., pp. 466-467). Oxford University Press. Since silyl electrophiles are HSAB theory, hard and silicon-oxygen bonds are very strong, the oxygen (of the carbonyl compound or enolate) acts as the nucleophile to form a Si-O single bond. The most commonly used silyl electrophile is trimethylsilyl chloride. To increase the rate of reaction, trimethylsilyl triflate may also be used in the place of trimethylsilyl chloride as a more el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylsilyl Compounds
A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom minus;Si(CH3)3 which is in turn bonded to the rest of a molecule. This structural group is characterized by chemical inertness and a large molecular volume, which makes it useful in a number of applications. A trimethylsilyl group bonded to a methyl group forms tetramethylsilane, which is abbreviated as TMS as well. Compounds with trimethylsilyl groups are not normally found in nature. Chemists sometimes use a trimethylsilylating reagent to derivatize rather non-volatile compounds such as certain alcohols, phenols, or carboxylic acids by substituting a trimethylsilyl group for a hydrogen in the hydroxyl groups on the compounds. This way trimethylsiloxy groups minus;O-Si(CH3)3are formed on the molecule. A couple of examples of trimethylsilylating agents include trimethylsilyl chloride and bis(trimethylsilyl)acetamide. Trimethy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


silyl.png)