|
Triiodide
In chemistry, triiodide usually refers to the triiodide ion, . This anion, one of the polyhalogen ions, is composed of three iodine atoms. It is formed by combining aqueous solutions of iodide salts and iodine. Some salts of the anion have been isolated, including thallium(I) triiodide (Tl+ 3sup>−) and ammonium triiodide ( H4sup>+ 3sup>−). Triiodide is observed to be a red colour in solution. Nomenclature Other chemical compounds with "triiodide" in their name may contain three iodide centers that are not bonded to each other as the triiodide ion, but exist instead as separate iodine atoms or iodide ions. Examples include nitrogen triiodide (NI3) and phosphorus triiodide (PI3), where individual iodine atoms are covalently bonded to a central atom. As some cations have the theoretical possibility to form compounds with both triiodide and iodide ions, such as ammonium, compounds containing iodide anions in a 3:1 stoichiometric ratio should only be referred to as triiodides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Triiodide
Nitrogen triiodide is an inorganic compound with the formula N I3. It is an extremely sensitive contact explosive: small quantities explode with a loud, sharp snap when touched even lightly, releasing a purple cloud of iodine vapor; it can even be detonated by alpha radiation. NI3 has a complex structural chemistry that is difficult to study because of the instability of the derivatives. Although nitrogen is more electronegative than iodine, the compound was so named due to its analogy to the compound nitrogen trichloride. Structure of NI3 and its derivatives Nitrogen triiodide was first characterized by Raman spectroscopy in 1990 when it was prepared by an ammonia-free route. Boron nitride reacts with iodine monofluoride in trichlorofluoromethane at −30 °C to produce pure NI3 in low yield: :BN + 3 IF → NI3 + BF3 NI3 is pyramidal (C3v molecular symmetry), as are the other nitrogen trihalides and ammonia. The material that is usually called "nitrogen triiodide" is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Triiodide
Phosphorus triiodide (PI3) is an inorganic compound with the formula PI3. A red solid, it is a common misconception that PI3 is too unstable to be stored; it is, in fact, commercially available. It is widely used in organic chemistry for converting alcohols to alkyl iodides. It is also a powerful reducing agent. Note that phosphorus also forms a lower iodide, P2I4, but the existence of PI5 is doubtful at room temperature. Properties PI3 has a low dipole moment in carbon disulfide solution, because the P-I bond has almost no dipole. The P-I bond is also weak; PI3 is much less stable than PBr3 and PCl3, with a standard enthalpy of formation for PI3 of only −46 kJ/ mol (solid). The phosphorus atom has an NMR chemical shift of 178 ppm (downfield of H3PO4). Reactions Phosphorus triiodide reacts vigorously with water, producing phosphorous acid (H3PO3) and hydroiodic acid (HI), along with smaller amounts of phosphine and various P-P-containing compounds. Alcohols likewise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thallium Triiodide
Thallium triiodide is a chemical compound of thallium and iodine with formula . Unlike the other thallium trihalides, which contain thallium(III), is a thallium(I) salt and contains the triiodide ion, . An appreciation as to why Tl+ is not oxidised to Tl3+ in the reaction: : Tl3+ + 2 I− → Tl+ + I2 can be gained by considering the standard reduction potentials of the half cells which are: : Tl3+ + 2 → Tl+;''Er''° = 1.252 : I2 + 2 → 2 I−;''Er''° = 0.5355 The favoured reaction is therefore the reduction of Tl3+ to Tl+ (1.252 > 0.5355). Using standard electrode potentials in this way must be done with caution as factors such as complex formation and solvation may affect the reaction. TlI3 is no exception as it is possible to stabilise thallium(III) with excess I− forming the ion (isoelectronic with the tetraiodomercurate anion and with lead(IV) iodide). Structure and preparation TlI3 is formulated , and has a similar structure to NH4I3, CsI3 an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thallium Triiodide
Thallium triiodide is a chemical compound of thallium and iodine with formula . Unlike the other thallium trihalides, which contain thallium(III), is a thallium(I) salt and contains the triiodide ion, . An appreciation as to why Tl+ is not oxidised to Tl3+ in the reaction: : Tl3+ + 2 I− → Tl+ + I2 can be gained by considering the standard reduction potentials of the half cells which are: : Tl3+ + 2 → Tl+;''Er''° = 1.252 : I2 + 2 → 2 I−;''Er''° = 0.5355 The favoured reaction is therefore the reduction of Tl3+ to Tl+ (1.252 > 0.5355). Using standard electrode potentials in this way must be done with caution as factors such as complex formation and solvation may affect the reaction. TlI3 is no exception as it is possible to stabilise thallium(III) with excess I− forming the ion (isoelectronic with the tetraiodomercurate anion and with lead(IV) iodide). Structure and preparation TlI3 is formulated , and has a similar structure to NH4I3, CsI3 an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek 'violet-coloured'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. It is the least abundant of the stable halogens, being the sixty-first most abundant element. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek 'violet-coloured'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. It is the least abundant of the stable halogens, being the sixty-first most abundant element. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Triiodide
Ammonium triiodide (NH4I3) is the salt (chemistry), salt of the ammonium cation with the triiodide anion. Sometimes the name ammonium triiodide is mistakenly used to refer to a different compound, nitrogen triiodide (NI3), or more precisely, the slightly more stable ammine, NI3 · NH3. References Ammonium compounds Polyhalides {{Inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Triiodide
Ammonium triiodide (NH4I3) is the salt (chemistry), salt of the ammonium cation with the triiodide anion. Sometimes the name ammonium triiodide is mistakenly used to refer to a different compound, nitrogen triiodide (NI3), or more precisely, the slightly more stable ammine, NI3 · NH3. References Ammonium compounds Polyhalides {{Inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Three-center Four-electron Bond
The 3-center 4-electron (3c–4e) bond is a model used to explain bonding in certain hypervalent molecules such as tetratomic and hexatomic interhalogen compounds, sulfur tetrafluoride, the xenon fluorides, and the bifluoride ion. It is also known as the Pimentel–Rundle three-center model after the work published by George C. Pimentel in 1951,Pimentel, G. C. The Bonding of Trihalide and Bifluoride Ions by the Molecular Orbital Method. ''J. Chem. Phys.'' 1951, ''19'', 446-448. which built on concepts developed earlier by Robert E. Rundle for electron-deficient bonding.Rundle, R. E. Electron Deficient Compounds. II. Relative Energies of "Half-Bonds". ''J. Chem. Phys.'' 1949, ''17'', 671–675. An extended version of this model is used to describe the whole class of hypervalent molecules such as phosphorus pentafluoride and sulfur hexafluoride as well as multi-center π-bonding such as ozone and sulfur trioxide. There are also molecules such as diborane (B2H6) and dialane (Al2H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
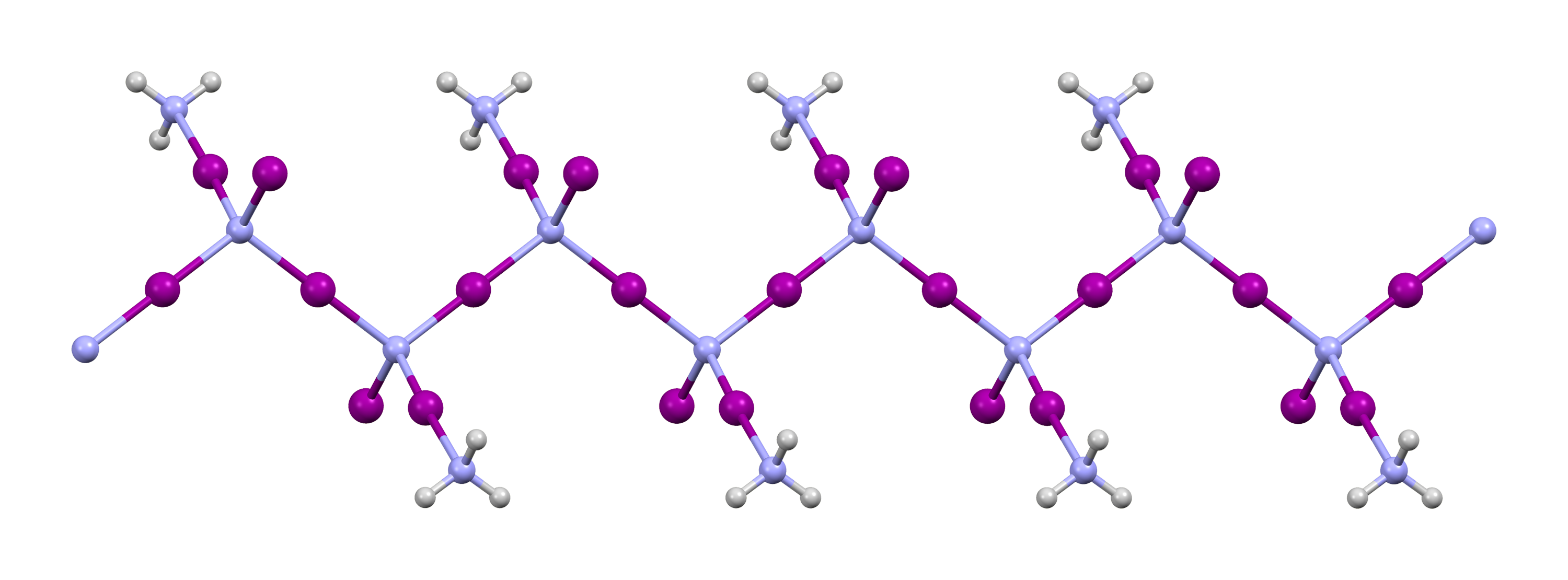
Polyiodide
The polyiodides are a class of polyhalogen anions composed entirely of iodine atoms. The most common and simplest member is the triiodide ion, . Other known larger polyiodides include 4sup>2−, 5sup>−, 6sup>2−, 7sup>−, 8sup>2−, 9sup>−, 10sup>2−, 10sup>4−, 11sup>3−, 12sup>2−, 13sup>3−, 14sup>4-, 16sup>2−, 22sup>4−, 26sup>3−, 26sup>4−, 28sup>4− and 29sup>3−. All these can be considered as formed from the interaction of the I–, I2, and building blocks. Preparation The polyiodides can be made by addition of stoichiometric amounts of I2 to solutions containing I− and , with the presence of large countercations to stabilize them. For example, KI3·H2O can be crystallized from a saturated solution of KI when a stoichiometric amount of I2 is added and cooled. Structure ] ] Polyiodides are characterized by their highly complex and variable structures, and can be considered as associations of I2, I−, and units. Discrete polyiod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium(III) Iodide
Gallium(III) iodide is the inorganic compound with the formula Ga I3. A yellow hygroscopic solid, it is the most common iodide of gallium. In the chemical vapor transport In chemistry, a chemical transport reaction describes a process for purification and crystallization of non- volatile solids. The process is also responsible for certain aspects of mineral growth from the effluent of volcanoes. The technique ... method of growing crystals of gallium arsenide uses iodine as the transport agent. In the solid state, it exists as the dimer Ga2I6. "Gallium(I) iodide" Gallium triiodide can be reduced with gallium metal to give a green-colored solid called "gallium(I) iodide." The nature of this species is unclear, but it is useful for the preparation of compounds of gallium(I) and gallium(II) and is reported as useful in organic syntheses.GaI: A new reagent for chemo- and diastereoselective C–C bond forming reactions, Green SP, Jones C., Stasch A., Rose R.P, New J. C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability. Structure and characteristics of inorganic iodides Iodide is one of the largest monatomic anions. It is assigned a radius of around 206 picometers. For comparison, the lighter halides are considerably smaller: bromide (196 pm), chloride (181 pm), and fluoride (133 pm). In part because of its size, iodide forms relatively weak bonds with most elements. Most iodide salts are soluble in water, but often less so than the related chlorides and bromides. Iodide, being large, is less hydrophilic compared to the smaller anions. One consequence of this is that sodium iodide is highly soluble in acetone, whereas sodium chloride is n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |