|
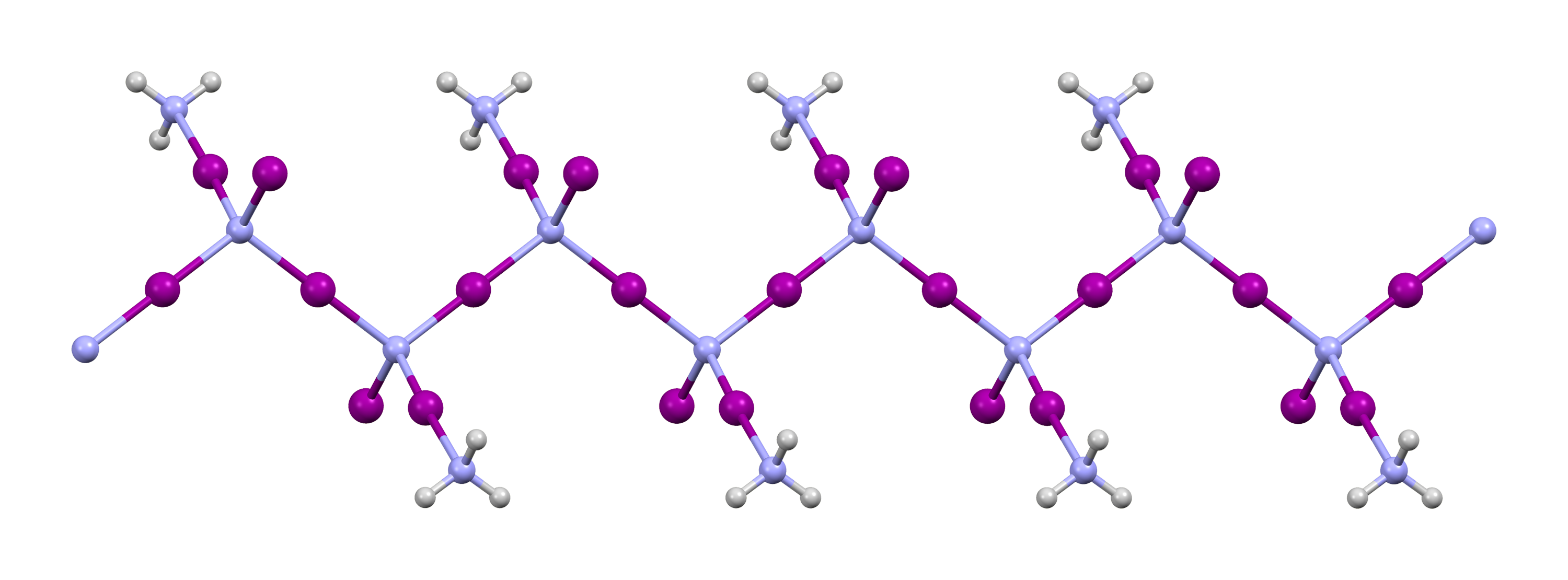
Nitrogen Triiodide
Nitrogen triiodide is an inorganic compound with the formula N I3. It is an extremely sensitive contact explosive: small quantities explode with a loud, sharp snap when touched even lightly, releasing a purple cloud of iodine vapor; it can even be detonated by alpha radiation. NI3 has a complex structural chemistry that is difficult to study because of the instability of the derivatives. Although nitrogen is more electronegative than iodine, the compound was so named due to its analogy to the compound nitrogen trichloride. Structure of NI3 and its derivatives Nitrogen triiodide was first characterized by Raman spectroscopy in 1990 when it was prepared by an ammonia-free route. Boron nitride reacts with iodine monofluoride in trichlorofluoromethane at −30 °C to produce pure NI3 in low yield: :BN + 3 IF → NI3 + BF3 NI3 is pyramidal (C3v molecular symmetry), as are the other nitrogen trihalides and ammonia. The material that is usually called "nitrogen triiodide" is pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general anesthetic, until non-flammable drugs were developed, such as halothane. It has been used as a recreational drug to cause intoxication. Production Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises. Vapor-phase dehydration of ethanol over some alumina catalysts can give diethyl ether yields of up to 95%. Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis. Ethanol is mixed with a stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oswald Silberrad
Oswald J. Silberrad (1878 – 17 June 1960) was a British chemist who specialised in explosives, the related field of dye stuffs, and metallurgy. Life and works Silberrad was born at Buckhurst Hill in Essex and was the younger brother of the writer Una Lucy Silberrad. He studied chemistry at the City and Guilds Technical College. From 1898 to 1900 he attended the University of Würzburg. On leaving Würzburg he worked at various positions until he joined the United Kingdom Government Explosives Committee. This committee had been set up after the Boer War to investigate the shortcomings of British explosives. In 1901, at the age of 23, Silberrad was appointed chemist to the committee. Later he took the position as head of the committee’s research institute at Woolwich. One of his contributions was to bring TNT into use as an explosive for Royal Navy ordnance, thus following a practice that was already well known in Germany. In 1906 he left the institute, and in 1907 found ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactive decay. Nuclear fission of heavy elements was discovered on Monday 19 December 1938, by German chemist Otto Hahn and his assistant Fritz Strassmann in cooperation with Austrian-Swedish physicist Lise Meitner. Hahn understood that a "burst" of the atomic nuclei had occurred. Meitner explained it theoretically in January 1939 along with her nephew Otto Robert Frisch. Frisch named the process by analogy with biological fission of living cells. For heavy nuclides, it is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place). Like nuclear fusion, for fission to produce energy, the total binding energy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Particles
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be produced in other ways. Alpha particles are named after the first letter in the Greek alphabet, α. The symbol for the alpha particle is α or α2+. Because they are identical to helium nuclei, they are also sometimes written as or indicating a helium ion with a +2 charge (missing its two electrons). Once the ion gains electrons from its environment, the alpha particle becomes a normal (electrically neutral) helium atom . Alpha particles have a net spin of zero. Due to the mechanism of their production in standard alpha radioactive decay, alpha particles generally have a kinetic energy of about 5 MeV, and a velocity in the vicinity of 4% of the speed of light. (See discussion below for the limits of these figures in alpha decay.) T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Detonation
Detonation () is a type of combustion involving a supersonic exothermic front accelerating through a medium that eventually drives a shock front propagating directly in front of it. Detonations propagate supersonically through shock waves with speeds in the range of 1 km/sec and differ from deflagrations which have subsonic flame speeds in the range of 1 m/sec. Detonations occur in both conventional solid and liquid explosives, as well as in reactive gases. The velocity of detonation in solid and liquid explosives is much higher than that in gaseous ones, which allows the wave system to be observed with greater detail (higher resolution). A very wide variety of fuels may occur as gases (e.g. hydrogen), droplet fogs, or dust suspensions. In addition to dioxygen, oxidants can include halogen compounds, ozone, hydrogen peroxide and oxides of nitrogen. Gaseous detonations are often associated with a mixture of fuel and oxidant in a composition somewhat below conventional flammabil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Thiosulfate
Sodium thiosulfate (sodium thiosulphate) is an inorganic compound with the formula . Typically it is available as the white or colorless pentahydrate, . The solid is an efflorescent (loses water readily) crystalline substance that dissolves well in water. Sodium thiosulfate is used in gold mining, water treatment, analytical chemistry, the development of silver-based photographic film and prints, and medicine. The medical uses of sodium thiosulfate include treatment of cyanide poisoning and pityriasis. It is on the World Health Organization's List of Essential Medicines. Uses Sodium thiosulfate is used predominantly in industry. For example, it is used to convert dyes to their soluble colorless forms, which are called leuco. It is also used to bleach "wool, cotton, silk, ...soaps, glues, clay, sand, bauxite, and... edible oils, edible fats, and gelatin." Medical uses Sodium thiosulfate is used in the treatment of cyanide poisoning. Other uses include topical treatment of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Iodide
Ammonium iodide is the chemical compound NH4I. It is used in photographic chemicals and some medications.Holleman, A. F.; Wiberg, E. ''Inorganic Chemistry'' Academic Press: San Diego, 2001. . It can be prepared by the action of hydroiodic acid on ammonia. It is easily soluble in water, from which it crystallizes in cubes. It is also soluble in ethanol. It gradually turns yellow on standing in moist air, owing to decomposition with liberation of iodine. Preparation Ammonium iodide can be made in lab by reacting ammonia or ammonium hydroxide with hydroiodic acid or hydrogen iodide gas: : NH3 + HI → NH4I : NH4OH + HI → NH4I + H2O It is also formed by the decomposition of ammoniated nitrogen triiodide Nitrogen triiodide is an inorganic compound with the formula N I3. It is an extremely sensitive contact explosive: small quantities explode with a loud, sharp snap when touched even lightly, releasing a purple cloud of iodine vapor; it can even b ... (an explosive). Referenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dynamite
Dynamite is an explosive made of nitroglycerin, sorbents (such as powdered shells or clay), and Stabilizer (chemistry), stabilizers. It was invented by the Swedish people, Swedish chemist and engineer Alfred Nobel in Geesthacht, Northern Germany, and patented in 1867. It rapidly gained wide-scale use as a more robust alternative to gun powder, black powder. History Dynamite was invented by Swedish chemist Alfred Nobel in the 1860s and was the first safely manageable explosive stronger than black powder. Alfred Nobel's father, Immanuel Nobel, was an industrialist, engineer, and inventor. He built bridges and buildings in Stockholm and founded Sweden's first rubber factory. His construction work inspired him to research new methods of blasting rock that were more effective than black powder. After some bad business deals in Sweden, in 1838 Immanuel moved Nobel family, his family to Saint Petersburg, where Alfred and his brothers were educated privately under Swedish and Russi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phlegmatizers
A phlegmatizer is a compound that minimizes the explosive tendency of compound or material. The term is derived from the word phlegmatic, meaning 'not easily excited'. Many chemical compounds that are potentially explosive have useful non-explosive applications. One large family of phlegmatizers are phthalate esters, which are used as solvents to minimize the explosive tendency of organic peroxides, such as dibenzoyl peroxide and MEKP, which are widely used initiators for polymerization In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer, monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are ...s.{{Ullmann , vauthors = Herbert K, Götz PH, Siegmeier R, Mayr W , title = Peroxy Compounds, Organic , doi = 10.1002/14356007.a19_199 References Liquid explosives Organic peroxide explosives ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitroglycerin
Nitroglycerin (NG), (alternative spelling of nitroglycerine) also known as trinitroglycerin (TNG), nitro, glyceryl trinitrate (GTN), or 1,2,3-trinitroxypropane, is a dense, colorless, oily, explosive liquid most commonly produced by nitrating glycerol with white fuming nitric acid under conditions appropriate to the formation of the nitric acid ester. Chemically, the substance is an organic nitrate compound rather than a nitro compound, but the traditional name is retained. Invented in 1847 by Ascanio Sobrero, nitroglycerin has been used ever since as an active ingredient in the manufacture of explosives, namely dynamite, and as such it is employed in the construction, demolition, and mining industries. Since the 1880s, it has been used by militaries as an active ingredient and gelatinizer for nitrocellulose in some solid propellants such as cordite and ballistite. It is a major component in double-based smokeless propellants used by reloaders. Combined with nitrocellulose, hund ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activation Energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Activation energy can be thought of as the magnitude of the potential barrier (sometimes called the energy barrier) separating minima of the potential energy surface pertaining to the initial and final thermodynamic state. For a chemical reaction to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. The term "activation energy" was introduced in 1889 by the Swedish scientist Svante Arrhenius. Other uses Although less commonly used, activation energy also applies to nuclear reactions and various other physical phenomena. Te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steric Effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel. Steric hindrance Steric hindrance is a consequence of steric effects. Steric hindrance is the slowing of chemical reactions due to steric bulk. It is usually manifested in ''intermolecular reactions'', whereas discussion of steric effects often focus on ''intramolecular interactions''. Steric hindrance is often exploited to control selectivity, such as slowing unwanted side-reactions. Steric hindrance between adjacent groups can also affect torsional ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |