|
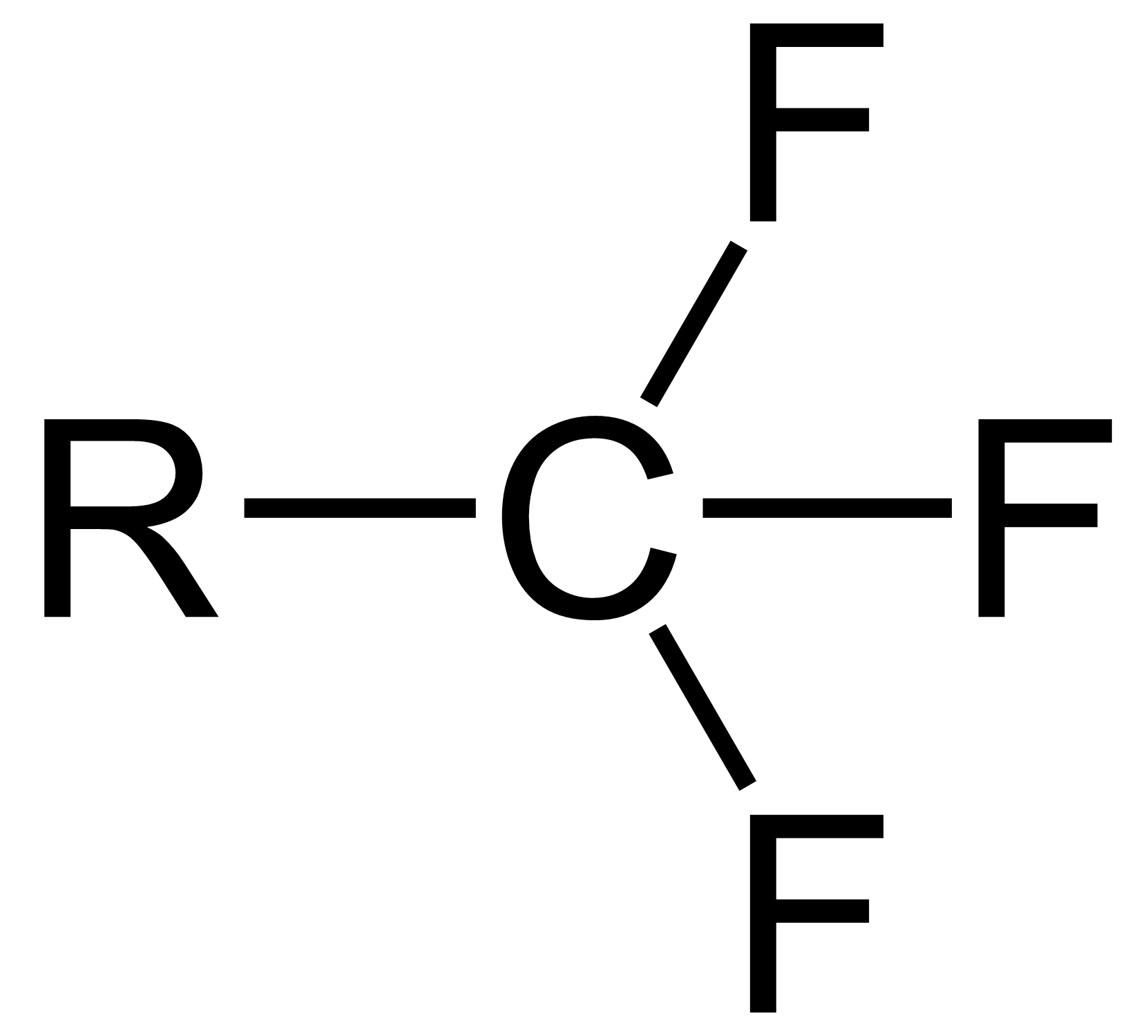
Trifluoromethyl Alcohol
Trifluoromethanol is the organic compound with the formula . It is also referred to as perfluoromethanol or trifluoromethyl alcohol. The compound is the simplest perfluoroalcohol. The substance is a colorless gas, which is unstable at room temperature. Synthesis Like all primary and secondary perfluoroalcohols, trifluoromethanol eliminates hydrogen fluoride in an endothermic reaction and forms carbonyl fluoride. : ⇌ + (I) At temperatures in the range of -120 °C, trifluoromethanol can be prepared from trifluoromethoxy chloride and hydrogen chloride: : + → + (II) In this reaction, the recombination of a partially positively charged chlorine atom (in trifluoromethoxy chloride) with a partially negatively charged chlorine atom (in hydrogen chloride) is used as elemental chlorine. The undesired products, by-products chlorine, hydrogen chloride, and chlorotrifluoromethane, can be removed by evaporation at -110 °C. Trifluoromethanol has a melting point of -82 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluoroalcohol
Fluoroalcohols are organofluorine compounds consisting of an alcohol functional group with at least one C-F bond. These compounds often have distinctive solvent properties. Perfluoroalcohols Most primary and secondary perfluoroalcohols are unstable, for example trifluoromethanol eliminates hydrogen fluoride, forming carbonyl fluoride. This reaction is reversible. : ⇌ + {{chem, H, F (I) Stable perfluorinated alcohols include nonafluoro-tert-butyl alcohol ((CF3)3COH) and pentafluorophenol (C6F5OH). Partially fluorinated alcohols Numerous partially fluorinated alcohols are known. Trifluoroethanol is a popular solvent. Fluorotelomer alcohols are precursors to perfluorocarboxylic acids. Pirkle's alcohol is used a chiral shift reagent in nuclear magnetic resonance spectroscopy Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an inorganic compound with the chemical formula . This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers, e.g. polytetrafluoroethylene (PTFE). HF is widely used in the petrochemical industry as a component of superacids. Hydrogen fluoride boils at near room temperature, much higher than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid had been known in the glass industry before then. French chemist Edmond Frémy (1814–1894) is credited with discoveri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Fluoride
Carbonyl fluoride is a chemical compound with the formula COF2. It is a carbon oxohalide. This gas, like its analog phosgene, is colourless and highly toxic. The molecule is planar with ''C''2v symmetry, bond lengths of 1.174 Å (C=O) and 1.312 Å (C–F), and an F–C–F bond angle of 108.0°. Preparation and properties Carbonyl fluoride is usually produced as a decomposition product of fluorinated hydrocarbons in the thermal decomposition thereof, for example from trifluoromethanol or tetrafluoromethane in the presence of water: : + → + 2 Carbonyl fluoride can also be prepared by reaction of phosgene with hydrogen fluoride and the oxidation of carbon monoxide, although the latter tends to result in over-oxidation to carbon tetrafluoride. The oxidation of carbon monoxide with silver difluoride is convenient: : + 2 → + 2 Carbonyl fluoride is unstable in the presence of water, hydrolyzing to carbon dioxide and hydrogen fluoride: : + → + 2 Safety Carbonyl fluor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride gas and hydrochloric acid are important in technology and industry. Hydrochloric acid, the aqueous solution of hydrogen chloride, is also commonly given the formula HCl. Reactions Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom H and a chlorine atom Cl connected by a polar covalent bond. The chlorine atom is much more electronegative than the hydrogen atom, which makes this bond polar. Consequently, the molecule has a large dipole moment with a negative partial charge (δ−) at the chlorine atom and a positive partial charge (δ+) at the hydrogen atom. In part because of its high polarity, HCl is very soluble in water (and in other polar solvents). Upon contact, and HCl combine to form hydronium cations and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorotrifluoromethane
Chlorotrifluoromethane, R-13, CFC-13, or Freon 13, is a non-flammable, non-corrosive chlorofluorocarbon (CFC) and also a mixed halomethane. It is a man-made substance used primarily as a refrigerant. When released into the environment, CFC-13 has a high ozone depletion potential, high global warming potential, and long atmospheric lifetime. Preparation It can be prepared by reacting carbon tetrachloride with hydrogen fluoride in the presence of a catalytic amount of antimony pentachloride: CCl4 + 3HF → CClF3 + 3HCl This reaction can also produce trichlorofluoromethane (CCl3F), dichlorodifluoromethane (CCl2F2) and tetrafluoromethane (CF4). Production phaseout Per the international Montreal Protocol, CFC-13 began a phase out and replacement with alternative substances starting in the early 1990s that will culminate in a global ban on its production. The atmospheric abundance of CFC-13 rose from 3.0 parts per trillion (ppt) in year 2010 to 3.3 ppt in year 2020 based ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, volatile, colourless, flammable liquid with a distinctive alcoholic odour similar to that of ethanol (potable alcohol). A polar solvent, methanol acquired the name wood alcohol because it was once produced chiefly by the destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl benzoate, anisole, peroxyacids, as well as a host of more specialised chemicals. Occurrence Small amounts of methanol are present in normal, healthy hu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoroantimonic Acid
Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride, containing various cations and anions (the simplest being and ). This substance is a superacid that can be over a billion times stronger than 100% pure sulfuric acid in terms of its protonating ability measured by Hammett function. It even protonates some hydrocarbons to afford pentacoordinate carbocations ( carbonium ions). Fluoroantimonic acid is corrosive. For example, it cannot be contained directly in glass carboys, as it attacks glass, but can be stored in containers lined with PTFE (Teflon). Chemical composition Fluoroantimonic acid is formed by combining hydrogen fluoride and antimony pentafluoride: :SbF5 + 2 HF + H2F+ The speciation (i.e., the inventory of components) of "fluoroantimonic acid" is complex. Spectroscopic measurements show that fluoroantimonic acid consists of a mixture of HF-solvated protons, –_(such_as_)._Thus,_the_formula_""_is_a_convenient_but_overs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Environ
Environ or environs may refer to: * Environ (Loft), a New York performance space * Ramboll Environ, or ''ENVIRON'', a consulting firm in Arlington, Virginia * ''Environs'' (journal), a student-run law review covering environmental subjects * Environs, or surroundings Surroundings are the area around a given physical or geographical point or place. The exact definition depends on the field. Surroundings can also be used in geography (when it is more precisely known as vicinity, or vicinage) and mathematics, a ..., the area around a given physical or geographical point or place See also * * * Environment (other) {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoroethanol
2,2,2-Trifluoroethanol is the organic compound with the formula CF3CH2OH. Also known as TFE or trifluoroethyl alcohol, this colourless, water-miscible liquid has a smell reminiscent of ethanol. Due to the electronegativity of the trifluoromethyl group, this alcohol exhibits a stronger acidic character compared to ethanol. Synthesis Trifluoroethanol is produced industrially by hydrogenation or the hydride reduction of derivatives of trifluoroacetic acid, such as the esters or acyl chloride. TFE can also be prepared by hydrogenolysis of compounds of generic formula CF3−CHOH−OR (where R is hydrogen or an alkyl group containing from one to eight carbon atoms), in the presence of a palladium containing catalyst deposited on activated charcoal. As a co-catalyst for this conversion tertiary aliphatic amines like triethylamine are commonly employed. Uses Trifluoroethanol is used as a solvent in organic chemistry. Oxidations of sulfur compounds using hydrogen peroxide are effectivel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoromethyl Compounds
The trifluoromethyl group is a functional group that has the formula -CF3. The naming of is group is derived from the methyl group (which has the formula -CH3), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane H–, 1,1,1-trifluoroethane –, and hexafluoroacetone –CO–. Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol. Uses The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized natural fluorocarbon based compounds. The medicinal use of the trifloromethyl group dates from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primary Alcohols
A primary alcohol is an alcohol in which the hydroxy group In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ... is bonded to a primary carbon atom. It can also be defined as a molecule containing a “–CH2OH” group. In contrast, a secondary alcohol has a formula “–CHROH” and a tertiary alcohol has a formula “–CR2OH”, where “R” indicates a carbon-containing group. Examples of primary alcohols include ethanol and n-Butanol, 1-butanol. Methanol is also generally regarded as a primary alcohol, including the 1911 edition of the Encyclopædia Britannica,. See also * Alcohol (chemistry), Alcohol (especially Nomenclature section for discussion on Secondary and Tertiary alcohols.) * Oxidation of primary alcohols to carboxylic acids References Primary alcohols, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |