|
Transition Metal Dithiocarbamate Complexes
133px, Tris(dithiocarbamate) complexes have idealized D3 symmetry. Transition metal dithiocarbamate complexes are coordination complexes containing one or more dithiocarbamate ligand, which are typically abbreviated R2dtc−. Many complexes are known. Several homoleptic derivatives have the formula M(R2dtc)n where n = 2 and 3. Ligand characteristics Dithiocarbamates are anions. Because of the pi-donor properties of the amino substituent, the two sulfur centers show enhanced basicity. This situation is represented by the zwitterionic resonance structure that depicts a positive charge on N and negative charges on both sulfurs. This N to C pi-bonding results in partial double bond character. Consequently, barriers to rotational about this bond are elevated. Another consequence of their high basicity, dithiocarbamates often stabilize complexes in uncharacteristically high oxidation state (e.g., Fe(IV), Co(IV), Ni(III), Cu(III)). Dithiocarbamate salts are easily synthesized. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiuram Disulfide
Thiuram disulfides are a class of organosulfur compounds with the formula (R2NCSS)2. Many examples are known, but popular ones include R = Me and Et. They are disulfides obtained by oxidation of the dithiocarbamates. These compounds are used in sulfur vulcanization of rubber as well as pesticides and drugs. They are typically white or pale yellow solids that are soluble in organic solvents. Preparation, structure, reactions They are prepared by the oxidation of the salts of the corresponding dithiocarbamates (e.g. sodium diethyldithiocarbamate). Typical oxidants are chlorine and hydrogen peroxide: :2 R2NCSSNa + Cl2 → (R2NCSS)2 + 2 NaCl Thiuram disulfides react with Grignard reagents to give esters of dithiocarbamic acid, as in the preparation of methyl dimethyldithiocarbamate: : e2NC(S)Ssub>2 + MeMgX → Me2NC(S)SMe + Me2NCS2MgX The compounds feature planar dithiocarbamate subunits and are linked by an S−S bond of 2.00 Å. The C(S)−N bond is sh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Tris(dimethyldithiocarbamate)
Iron tris(dimethyldithiocarbamate) is the coordination complex of iron with dimethyldithiocarbamate with the formula Fe(S2CNMe2)3 (Me = methyl). It is marketed as a fungicide. Synthesis, structure, bonding Iron tris(dithiocarbamate)s are typically are prepared by salt metathesis reactions. Iron tris(dimethyldithiocarbamate) is an octahedral coordination complex of iron(III) with D3 symmetry. Spin crossover (SCO) was first observed in 1931 by Cambi ''et al.'' who discovered anomalous magnetic behavior for the tris(N,N-dialkyldithiocarbamatoiron(III) complexes. The spin states of these complexes are sensitive to the nature of the amine substituents. Iron tris(dithiocarbamate)s characteristically react with nitric oxide to give Fe(dtc)2NO. This efficient chemical trapping reaction provides a means to detect NO. Reflecting the strongly donating properties of dithiocarbamate ligands, iron tris(dithiocarbamate)s oxidize at relatively mild potentials to give isolable iron(IV) deri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vulcanization Accelerator
Sulfur vulcanization is a chemical process for converting natural rubber or related polymers into materials of varying hardness, elasticity, and mechanical durability by heating them with sulfur or sulfur-containing compounds. Sulfur forms cross-linking bridges between sections of polymer chains which affects the mechanical and electronic properties. Many products are made with vulcanized rubber, including tires, shoe soles, hoses, and conveyor belts. The term is derived from Vulcan, the Roman god of fire. The main polymers subjected to sulfur vulcanization are polyisoprene (natural rubber, NR), polybutadiene rubber (BR) and styrene-butadiene rubber (SBR), and ethylene propylene diene monomer rubber ( EPDM rubber). All of these materials contain alkene groups adjacent to methylene groups. Other specialty rubbers may also be vulcanized, such as nitrile rubber (NBR) and butyl rubber (IIR). Vulcanization, in common with the curing of other thermosetting polymers, is generally ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zineb
Zineb is the chemical compound with the formula n. Structurally, it is classified as a coordination polymer and a dithiocarbamate complex. This pale yellow solid is used as fungicide. Production and applications It is produced by treating ethylene bis(dithiocarbamate) sodium salt, "nabam", with zinc sulfate. This procedure can be carried out by mixing nabam and zinc sulfate in a spray tank.Michael A. Kamrin, (1997) Pesticide Profiles: Toxicity, Environmental Impact, and Fate, CRC Press, Its uses include control of downy mildews, rusts, and redfire disease. In the US it was once registered as a "General Use Pesticide", however all registrations were voluntarily cancelled following an EPA special review. It continues to be used in many other countries. Structure Zineb is a polymeric complex of zinc with a dithiocarbamate. The polymer is composed of Zn(dithiocarbamate)2 subunits linked by an ethylene (-CH2CH2-) backbone. A reference compound is n(S2CNEt2)2sub>2, which features ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferbam
Iron tris(dimethyldithiocarbamate) is the coordination complex of iron with dimethyldithiocarbamate with the formula Fe(S2CNMe2)3 (Me = methyl). It is marketed as a fungicide. Synthesis, structure, bonding Iron tris(dithiocarbamate)s are typically are prepared by salt metathesis reactions. Iron tris(dimethyldithiocarbamate) is an octahedral coordination complex of iron(III) with D3 symmetry. Spin crossover (SCO) was first observed in 1931 by Cambi ''et al.'' who discovered anomalous magnetic behavior for the tris(N,N-dialkyldithiocarbamatoiron(III) complexes. The spin states of these complexes are sensitive to the nature of the amine substituents. Iron tris(dithiocarbamate)s characteristically react with nitric oxide to give Fe(dtc)2NO. This efficient chemical trapping reaction provides a means to detect NO. Reflecting the strongly donating properties of dithiocarbamate ligands, iron tris(dithiocarbamate)s oxidize at relatively mild potentials to give isolable iron(IV) deri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Herbicide
Herbicides (, ), also commonly known as weedkillers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page for EPA reports on pesticide use ihere Selective herbicides control specific weed species, while leaving the desired crop relatively unharmed, while non-selective herbicides (sometimes called total weedkillers in commercial products) can be used to clear waste ground, industrial and construction sites, railways and railway embankments as they kill all plant material with which they come into contact. Apart from selective/non-selective, other important distinctions include ''persistence'' (also known as ''residual action'': how long the product stays in place and remains active), ''means of uptake'' (whether it is absorbed by above-ground foliage only, through the roots, or by other means), and ''mechanism of action'' (how it works). Historica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
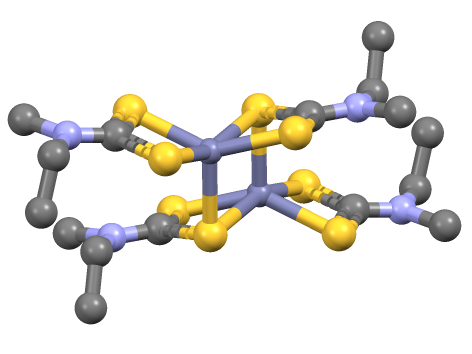
(Ru2(dtc)5)+ Isomers
''Ru, ru, or RU may refer to: Russia * Russia (ISO 3166-1 alpha-2 country code) * Russian language (ISO 639 alpha-2 code) * .ru, the Internet country code top-level domain for Russia China * Rù (入), the entering tone in Chinese language phonetics * Rú (儒), a Chinese language term for Confucianism * Ru (surname) (茹), a Chinese surname * Ru River (汝), in Henan, China * Ru ware, a type of Chinese pottery Educational institutions * Radboud University Nijmegen, in Nijmegen, Netherlands * Radford University, in Virginia, USA * Rai University in Gujarat, India * Rajshahi University in Bangladesh * Rama University in India * Ramkhamhaeng University in Thailand * Regis University in Colorado, USA * Reykjavík University Iceland * Rhodes University in Grahamstown, South Africa * Rockefeller University in New York, USA * Rockhurst University in Missouri, USA * Roosevelt University in Chicago, Illinois, USA * Rowan University in New Jersey, USA * Ruse University in Bu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Bis(dimethyldithiocarbamate)
Zinc dimethyldithiocarbamate is a coordination complex of zinc with dimethyldithiocarbamate. It is a pale yellow solid that is used as a fungicide, the sulfur vulcanization of rubber, and other industrial applications. Applications Known as ziram in agriculture, it was introduced in the United States in 1960 as a broad-spectrum fungicide. It was used to address scab on apples and pears, leaf curl in peaches, and anthracnose and blight in tomatoes. In 1981, additional uses for ziram were approved, including the prevention of leaf blight and scab on almonds, shot-hole in apricots, brown rot and leaf spot in cherries, and scab and anthracnose in pecans. Ziram also began to be used on residential ornaments as a bird and mammal repellent. As a protectant fungicide, it active on the plant’s surface where it forms a chemical barrier between the plant and a fungus. A protectant fungicide is not absorbed into the plant and must be applied prior to infection. Ziram can either be dire ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cobalt Tris(diethyldithiocarbamate)
Cobalt tris(diethyldithiocarbamate) is the coordination complex of cobalt with diethyldithiocarbamate with the formula Co(S2CNEt2)3 (Et = ethyl). It is a diamagnetic green solid that is soluble in organic solvents. Synthesis, structure, bonding Cobalt tris(dithiocarbamate)s are typically are prepared by air-oxidation of mixtures of dithiocarbamate salts and a cobalt(II) nitrate. Cobalt tris(diethyldithiocarbamate) is an octahedral coordination complex of low-spin Co(III) with idealized D3 symmetry. The Co-S distances are 267 pm. Reactions Oxidation of Co(Et2dtc)3 occurs at mild potentials to give the cobalt(IV) derivative. Treatment of Co(Et2dtc)3 with fluoroboric acid Fluoroboric acid or tetrafluoroboric acid (archaically, fluoboric acid) is an inorganic compound with the chemical formula +BF4−], where H+ represents the solvated proton. The solvent can be any suitably Lewis-basic entity. For instance, in w ... results in the removal of 0.5 equiv of ligand giving a binucle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Tris(diethyldithiocarbamate)
Iron tris(diethyldithiocarbamate) is the coordination complex of iron with diethyldithiocarbamate with the formula Fe(S2CNEt2)3 (Et = ethyl). It is a black solid that is soluble in organic solvents. Synthesis, structure, bonding Iron tris(dithiocarbamate)s are typically are prepared by salt metathesis reactions. Iron tris(diethyldithiocarbamate) is an octahedral coordination complex of iron(III) with idealized D3 symmetry. The phenomenon of spin crossover (SCO) was first reported in 1931 by Cambi ''et al.'' who observed anomalous magnetic behavior for the tris(N,N-dialkyldithiocarbamatoiron(III) complexes. The magnetism of these complexes are sensitive to the nature of the amine substituents as well as to temperature. This behavior is consistent with an equilibrium between two or more spin states. In the case of Fe(Et2dtc)3, X-ray crystallography reveals that the Fe-S bonds are 231 pm at 79K but 356 pm at 297 K. These data indicate a low-spin configuration at low temperatures an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel Bis(dimethyldithiocarbamate)
Nickel bis(dimethyldithiocarbamate) is the coordination complex on nickel and dimethyldithiocarbamate, with the formula Ni(S2CNMe2)2 (Me = methyl). It is the prototype for a large number of bis(dialkhyldithiocarbamate)s of nickel(II), which feature diverse organic substituents, all of which have similar structures. Nickel bis(dimethyldithiocarbamate) has been marketed as a fungicide and related complexes are used as stabilizers in polymers. Preparation and structure The compound precipitates as a black solid upon combining aqueous solutions of salts of nickel(II) and dimethyldithiocarbamate. In terms of structure and bonding the nickel is square planar, and the complex is diamagnetic.{{cite journal, title=The Chemistry of the Dithioacid and 1,1-Dithiolate Complexes, journal=Progress in Inorganic Chemistry, volume=11, author=D. Coucouvanis, pages=233–371, doi=10.1002/9780470166123.ch4 See also * Zinc dimethyldithiocarbamate - a related compound where nickel has been replaced w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |