|
Toxicated
Toxication, toxification or toxicity exaltation is the conversion of a chemical compound into a more toxic form in living organisms or in substrates such as soil or water. The conversion can be caused by enzymatic metabolism in the organisms, as well as by abiotic chemical reactions. While the parent drug are usually less active, both the parent drug and its metabolite can be chemically active and cause toxicity, leading to mutagenesis, teratogenesis, and carcinogenesis. Different classes of enzymes, such as P450-monooxygenases, epoxide hydrolase, or acetyltransferases can catalyze the process in the cell, mostly in the liver. Parent non-toxic chemicals are generally referred to as ''protoxins''. While toxication is generally undesirable, in certain cases it is required for the ''in vivo'' conversion of a prodrug to a metabolite with desired pharmacological or toxicological activity. Codeine is an example of a prodrug, metabolized in the body to the active compounds morphine and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, usi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
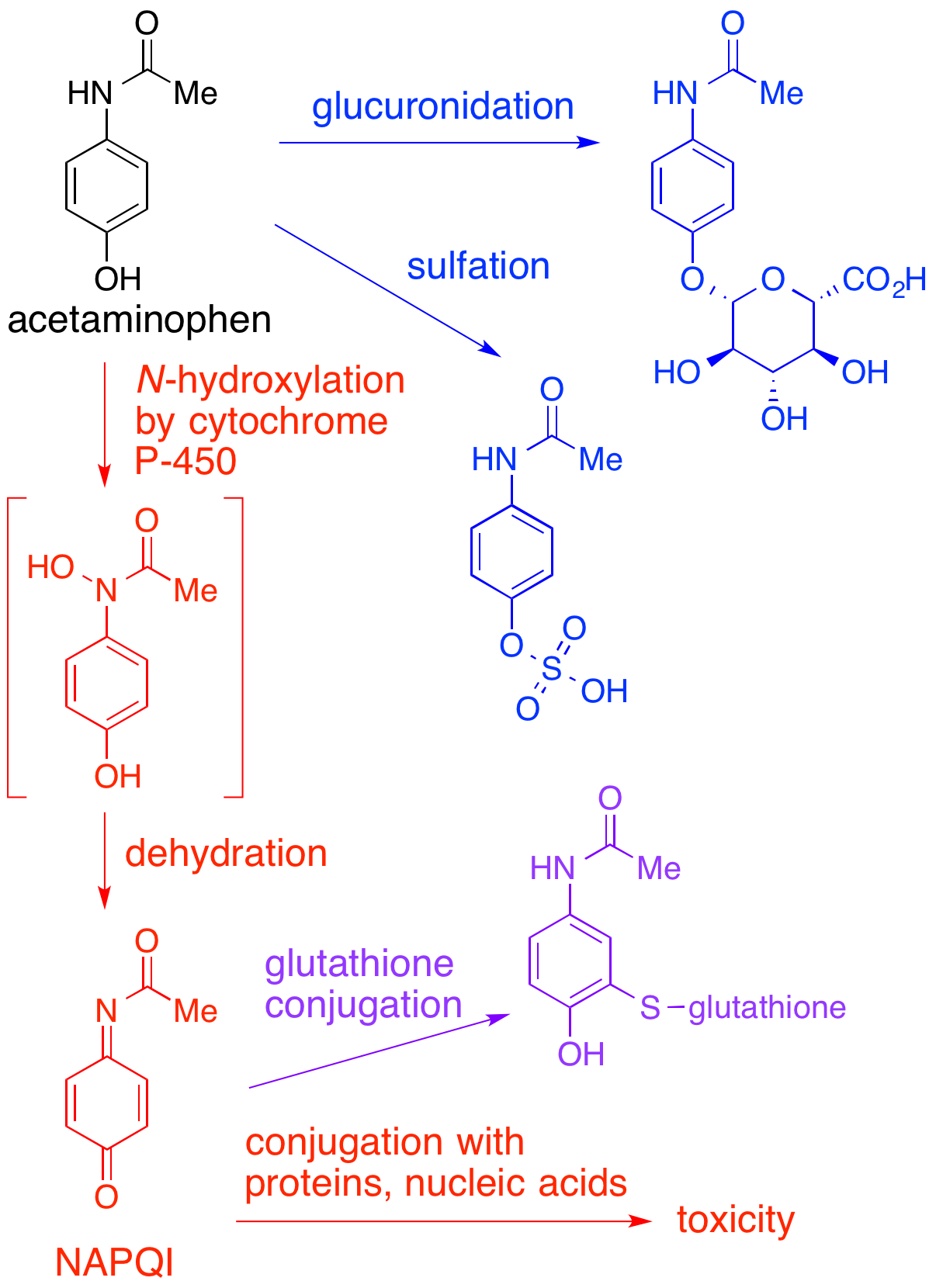
NAPQI
NAPQI, also known as NAPBQI or ''N''-acetyl-''p''-benzoquinone imine, is a toxic byproduct produced during the xenobiotic metabolism of the analgesic paracetamol (acetaminophen). It is normally produced only in small amounts, and then almost immediately detoxified in the liver. However, under some conditions in which NAPQI is not effectively detoxified (usually in the case of paracetamol overdose), it causes severe damage to the liver. This becomes apparent 3–4 days after ingestion and may result in death from fulminant liver failure several days after the overdose. Metabolism In adults, the primary metabolic pathway for paracetamol is glucuronidation. This yields a relatively non-toxic metabolite, which is excreted into bile and passed out of the body. A small amount of the drug is metabolized via the cytochrome P-450 pathway (to be specific, CYP3A4 and CYP2E1) into NAPQI, which is extremely toxic to liver tissue, as well as being a strong biochemical oxidizer. In an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Glycol Toxication
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds). Ethylene is widely used in the chemical industry, and its worldwide production (over 150 million tonnes in 2016) exceeds that of any other organic compound. Much of this production goes toward polyethylene, a widely used plastic containing polymer chains of ethylene units in various chain lengths. Ethylene is also an important natural plant hormone and is used in agriculture to force the ripening of fruits. The hydrate of ethylene is ethanol. Structure and properties This hydrocarbon has four hydrogen atoms bound to a pair of carbon atoms that are connected by a double bond. All six atoms that comprise ethylene are coplanar. The H-C-H angle is 117.4°, close to the 120° for ideal sp² hybridized carbon. The molecule is also relatively weak: rotat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxalic Acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and formula . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus '' Oxalis'', commonly known as wood-sorrels. It occurs naturally in many foods. Excessive ingestion of oxalic acid or prolonged skin contact can be dangerous. Oxalic acid has much greater acid strength than acetic acid. It is a reducing agent and its conjugate base, known as oxalate (), is a chelating agent for metal cations. Typically, oxalic acid occurs as the dihydrate with the formula . History The preparation of salts of oxalic acid (crab acid) from plants had been known, at least since 1745, when the Dutch botanist and physician Herman Boerhaave isolated a salt from wood sorrel. By 1773, François Pierre Savary of Fribourg, Switzerland had isolated oxalic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycolic Acid
Glycolic acid (or hydroxyacetic acid; chemical formula HOCH2CO2H) is a colorless, odorless and hygroscopic crystalline solid, highly soluble in water. It is used in various skin-care products. Glycolic acid is widespread in nature. A glycolate (sometimes spelled "glycollate") is a salt or ester of glycolic acid. History The name "glycolic acid" was coined in 1848 by French chemist Auguste Laurent (1807–1853). He proposed that the amino acid glycine—which was then called ''glycocolle''—might be the amine of a hypothetical acid, which he called "glycolic acid" (''acide glycolique''). Glycolic acid was first prepared in 1851 by German chemist Adolph Strecker (1822–1871) and Russian chemist Nikolai Nikolaevich Sokolov (1826–1877). They produced it by treating hippuric acid with nitric acid and nitrogen dioxide to form an ester of benzoic acid and glycolic acid (C6H5C(=O)OCH2COOH), which they called "benzoglycolic acid" (''Benzoglykolsäure''; also benzoyl glycolic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Glycol
Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odorless, colorless, flammable, viscous liquid. Ethylene glycol has a sweet taste, but it is toxic in high concentrations. Production Industrial routes Ethylene glycol is produced from ethylene (ethene), via the intermediate ethylene oxide. Ethylene oxide reacts with water to produce ethylene glycol according to the chemical equation: This reaction can be catalyzed by either acids or bases, or can occur at neutral pH under elevated temperatures. The highest yields of ethylene glycol occur at acidic or neutral pH with a large excess of water. Under these conditions, ethylene glycol yields of 90% can be achieved. The major byproducts are the oligomers diethylene glycol, triethylene glycol, and tetraethylene glycol. The separation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol Conversion
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, volatile, colourless, flammable liquid with a distinctive alcoholic odour similar to that of ethanol (potable alcohol). A polar solvent, methanol acquired the name wood alcohol because it was once produced chiefly by the destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl benzoate, anisole, peroxyacids, as well as a host of more specialised chemicals. Occurrence Small amounts of methanol are present in normal, healthy huma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Optic Nerve
In neuroanatomy, the optic nerve, also known as the second cranial nerve, cranial nerve II, or simply CN II, is a paired cranial nerve that transmits visual information from the retina to the brain. In humans, the optic nerve is derived from optic stalks during the seventh week of development and is composed of retinal ganglion cell axons and glial cells; it extends from the optic disc to the optic chiasma and continues as the optic tract to the lateral geniculate nucleus, pretectal nuclei, and superior colliculus. Structure The optic nerve has been classified as the second of twelve paired cranial nerves, but it is technically part of the central nervous system, rather than the peripheral nervous system because it is derived from an out-pouching of the diencephalon ( optic stalks) during embryonic development. As a consequence, the fibers of the optic nerve are covered with myelin produced by oligodendrocytes, rather than Schwann cells of the peripheral nervous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acidosis
Acidosis is a process causing increased acidity in the blood and other body tissues (i.e., an increase in hydrogen ion concentration). If not further qualified, it usually refers to acidity of the blood plasma. The term ''acidemia'' describes the state of low blood pH, while ''acidosis'' is used to describe the processes leading to these states. Nevertheless, the terms are sometimes used interchangeably. The distinction may be relevant where a patient has factors causing both acidosis and alkalosis, wherein the relative severity of both determines whether the result is a high, low, or normal pH. Acidemia is said to occur when arterial pH falls below 7.35 (except in the fetus – see below), while its counterpart (alkalemia) occurs at a pH over 7.45. Arterial blood gas analysis and other tests are required to separate the main causes. The rate of cellular metabolic activity affects and, at the same time, is affected by the pH of the body fluids. In mammals, the normal pH of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formaldehyde
Formaldehyde ( , ) ( systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section Forms below), hence it is stored as an aqueous solution (formalin), which is also used to store animal specimens. It is the simplest of the aldehydes (). The common name of this substance comes from its similarity and relation to formic acid. Formaldehyde is an important precursor to many other materials and chemical compounds. In 1996, the installed capacity for the production of formaldehyde was estimated at 8.7 million tons per year. It is mainly used in the production of industrial resins, e.g., for particle board and coatings. Forms Formaldehyde is more complicated than many simple carbon compounds in that it adopts several diverse forms. These compounds can often be used interchangeably and can be interconverted. *Molecular for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formic Acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure . It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Esters, salts and the anion derived from formic acid are called formates. Industrially, formic acid is produced from methanol. Natural occurrence In nature, formic acid is found in most ants and in stingless bees of the genus '' Oxytrigona''. Wood ants from the genus '' Formica'' can spray formic acid on their prey or to defend the nest. The puss moth caterpillar (''Cerura vinula'') will spray it as well when threatened by predators. It is also found in the trichomes of stinging nettle (''Urtica dioica''). Apart from that, this acid is incorporated in many fruits such as pineapple (0.21mg per 100g), apple (2mg per 100g) and kiwi (1mg per 100g), as well as in many vegetables, namely onion (45mg per 100g), eggplant (1.34 mg per 100g) an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Central Nervous System Depression
Central nervous system (CNS) depression is a physiological state that can result in a decreased rate of breathing, decreased heart rate, and loss of consciousness possibly leading to coma or death. It is the result of inhibited or suppressed brain activity. Causes Depression of the central nervous system is generally caused by the use of depressant drugs such as ethanol, opioids, barbiturates, benzodiazepines, general anesthetics, and anticonvulsants such as pregabalin used to treat epilepsy. Drug overdose is often caused by combining two or more depressant drugs, although overdose is also possible by consuming a large dose of one depressant drug. Central nervous system depression can also be caused by the accidental or intentional inhalation or ingestion of certain volatile chemicals such as butanone (contained in plastic cement) or isopropyl alcohol. Other causes of central nervous system depression are metabolic disturbances such as hypoglycaemia. Comparison In a s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |