|
Thermodynamic Diagram
Thermodynamic diagrams are diagrams used to represent the thermodynamic states of a material (typically fluid) and the consequences of manipulating this material. For instance, a temperature–entropy diagram (Temperature–entropy diagram, T–s diagram) may be used to demonstrate the behavior of a fluid as it is changed by a compressor. Overview Especially in meteorology they are used to analyze the actual state of the atmosphere derived from the measurements of radiosondes, usually obtained with weather balloons. In such diagrams, temperature and humidity values (represented by the dew point) are displayed with respect to atmospheric pressure, pressure. Thus the diagram gives at a first glance the actual atmospheric stratification and vertical water vapor distribution. Further analysis gives the actual base and top height of cumulus cloud, convective clouds or possible instabilities in the stratification. By assuming the energy amount due to solar radiation it is possible ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Thermodynamic Properties
In thermodynamics, a physical property is any property that is measurable, and whose value describes a state of a physical system. Thermodynamic properties are defined as characteristic features of a system, capable of specifying the system's state. Some constants, such as the ideal gas constant, , do not describe the state of a system, and so are not properties. On the other hand, some constants, such as (the freezing point depression constant, or cryoscopic constant), depend on the identity of a substance, and so may be considered to describe the state of a system, and therefore may be considered physical properties. "Specific" properties are expressed on a per mass basis. If the units were changed from per mass to, for example, per mole, the property would remain as it was (i.e., intensive or extensive). Regarding work and heat Work and heat are not thermodynamic properties, but rather '' process quantities:'' flows of energy across a system boundary. Systems do not ''con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Meter
The metre (British spelling) or meter (American spelling; see spelling differences) (from the French unit , from the Greek noun , "measure"), symbol m, is the primary unit of length in the International System of Units (SI), though its prefixed forms are also used relatively frequently. The metre was originally defined in 1793 as one ten-millionth of the distance from the equator to the North Pole along a great circle, so the Earth's circumference is approximately km. In 1799, the metre was redefined in terms of a prototype metre bar (the actual bar used was changed in 1889). In 1960, the metre was redefined in terms of a certain number of wavelengths of a certain emission line of krypton-86. The current definition was adopted in 1983 and modified slightly in 2002 to clarify that the metre is a measure of proper length. From 1983 until 2019, the metre was formally defined as the length of the path travelled by light in a vacuum in of a second. After the 2019 rede ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emagram
An emagram is one of four thermodynamic diagrams used to display temperature lapse rate and moisture content profiles in the atmosphere. The emagram has axes of temperature (T) and pressure (p). In the emagram, the dry adiabats make an angle of about 45 degrees with the isobars, isotherms are vertical and isopleths of saturation mixing ratio are almost straight and vertical. Usually, temperature and dew point data from radiosondes are plotted on these diagrams to allow calculations of convective stability or Convective Available Potential Energy. Wind barbs are often plotted at the side of a tephigram to indicate the winds at different heights. First devised in 1884 by Heinrich Hertz, the emagram is used primarily in Europe Europe is a large peninsula conventionally considered a continent in its own right because of its great physical size and the weight of its history and traditions. Europe is also considered a Continent#Subcontinents, subcontinent of Eurasia ...an coun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tephigram
A tephigram is one of four thermodynamic diagrams commonly used in weather analysis and forecasting. The name evolved from the original name "T-\phi-gram" to describe the axes of temperature (T) and entropy (\phi) used to create the plot. Usually, temperature and dew point data from radiosondes are plotted on these diagrams to allow calculations of convective stability or convective available potential energy (CAPE). Wind barbs are often plotted at the side of a tephigram to indicate the winds at different heights. Description The tephigram was invented by Napier Shaw in 1915 and is used primarily in the United Kingdom and Canada. Other countries use similar thermodynamic diagrams for the same purpose however the details of their construction vary. In the tephigram, isotherms are straight and have a 45 degree inclination to the right while isobars are horizontal and have a slight curve. Dry adiabats are also straight and have a 45 degree inclination to the left while moist a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
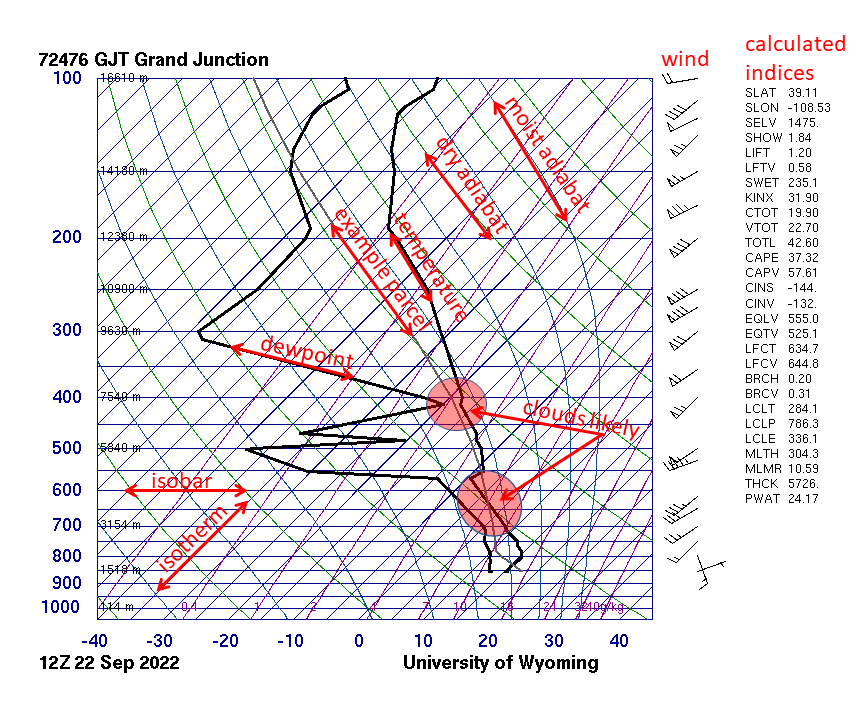
Skew-T Log-P Diagram
A skew-T log-P diagram is one of four thermodynamic diagrams commonly used in weather analysis and forecasting. In 1947, N. Herlofson proposed a modification to the emagram that allows straight, horizontal isobars and provides for a large angle between isotherms and dry adiabats, similar to that in the tephigram. It was thus more suitable for some of the newer analysis techniques being invented by the United States Air Force. Such a diagram has pressure plotted on the vertical axis, with a logarithmic scale (thus the "log-P" part of the name), and the temperature plotted skewed, with isothermal lines at 45° to the plot (thus the "skew-T" part of the name). Plotting a hypothetical set of measurements with constant temperature for all altitudes would result in a line angled 45° to the right. In practice, since temperature usually drops with altitude, the graphs are usually mostly vertical (see examples linked to below). The major use for skew-T log-P diagrams is the plotting of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maxwell's Thermodynamic Surface
Maxwell’s thermodynamic surface is an 1874 sculpture made by Scottish physicist James Clerk Maxwell (1831–1879). This model provides a three-dimensional space of the various states of a fictitious substance with water-like properties. This plot has coordinates volume (x), entropy (y), and energy (z). It was based on the American scientist Josiah Willard Gibbs’ graphical thermodynamics papers of 1873. The model, in Maxwell's words, allowed "the principal features of known substances obe represented on a convenient scale." Construction of the model Gibbs' papers defined what Gibbs called the "thermodynamic surface," which expressed the relationship between the volume, entropy, and energy of a substance at different temperatures and pressures. However, Gibbs did not include any diagrams of this surface. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturation Vapor Curve
The saturation vapor curve is the curve separating the two-phase state and the superheated vapor state in the T–s diagram ( temperature–entropy diagram). The '' saturated liquid curve'' is the curve separating the subcooled liquid state and the two-phase state in the T–s diagram. at LearnThermo.com When used in a power cycle, the fluid expansion depends strongly on the nature of this saturation curve: *A "wet" fluid shows a negative saturation vapor curve. If overheating before the expansion is limited, a two-phase state is obtained at the end of the expansion. : [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indicator Diagram
An indicator diagram is a chart used to measure the thermal, or cylinder, performance of reciprocating steam and internal combustion engines and compressors. An indicator chart records the pressure in the cylinder versus the volume swept by the piston, throughout the two or four strokes of the piston which constitute the engine, or compressor, cycle. The indicator diagram is used to calculate the work done and the power produced in an engine cylinder or used in a compressor cylinder. The indicator diagram was developed by James Watt and his employee John Southern to help understand how to improve the efficiency of steam engines. In 1796, Southern developed the simple, but critical, technique to generate the diagram by fixing a board so as to move with the piston, thereby tracing the "volume" axis, while a pencil, attached to a pressure gauge, moved at right angles to the piston, tracing "pressure". The gauge enabled Watt to calculate the work done by the steam while ensuring t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cooling Curve
A cooling curve is a line graph that represents the change of phase of matter, typically from a gas to a solid or a liquid to a solid. The independent variable (X-axis) is time and the dependent variable (Y-axis) is temperature.Garland, Nibler, and Shoemaker. Experiments in Physical Chemistry (7th ed.) Below is an example of a cooling curve used in castings. The initial point of the graph is the starting temperature of the matter, here noted as the "pouring temperature". When the phase change occurs, there is a "thermal arrest"; that is, the temperature stays constant. This is because the matter has more internal energy as a liquid or gas than in the state that it is cooling to. The amount of energy required for a phase change is known as latent heat Latent heat (also known as latent energy or heat of transformation) is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process — usually a first-order phase transition. Latent h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psychrometric Chart
Psychrometrics (or psychrometry, ; also called hygrometry) is the field of engineering concerned with the physical and thermodynamic properties of gas-vapor mixtures. Common applications Although the principles of psychrometry apply to any physical system consisting of gas-vapor mixtures, the most common system of interest is the mixture of water vapor and air, because of its application in heating, ventilation, and air-conditioning and meteorology. In human terms, our thermal comfort is in large part a consequence of not just the temperature of the surrounding air, but (because we cool ourselves via perspiration) the extent to which that air is saturated with water vapor. Many substances are hygroscopic, meaning they attract water, usually in proportion to the relative humidity or above a critical relative humidity. Such substances include cotton, paper, cellulose, other wood products, sugar, calcium oxide (burned lime) and many chemicals and fertilizers. Industries that us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mollier Diagram
Mollier may refer to: * Richard Mollier Richard Mollier (; 30 November 1863, Triest – 13 March 1935, Dresden) was a German professor of Applied Physics and Mechanics in Göttingen and Dresden, a pioneer of experimental research in thermodynamics, particularly for water, steam, and moi ..., German professor of Applied Physics and Mechanics ; * Louis-Marie Mollier, French-American pioneer priest of north-central Kansas ; * Jean-Yves Mollier, French Contemporary History teacher. {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




.jpg)