|
Thermal Expansion Coefficient
Thermal expansion is the tendency of matter to change its shape, area, volume, and density in response to a change in temperature, usually not including phase transitions. Temperature is a monotonic function of the average molecular kinetic energy of a substance. When a substance is heated, molecules begin to vibrate and move more, usually creating more distance between themselves. Substances which contract with increasing temperature are unusual, and only occur within limited temperature ranges (see examples below). The relative expansion (also called strain) divided by the change in temperature is called the material's coefficient of linear thermal expansion and generally varies with temperature. As energy in particles increases, they start moving faster and faster weakening the intermolecular forces between them, therefore expanding the substance. Overview Predicting expansion If an equation of state is available, it can be used to predict the values of the thermal expan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Material Properties (thermodynamics)
The thermodynamic properties of materials are intensive thermodynamic parameters which are specific to a given material. Each is directly related to a second order differential of a thermodynamic potential. Examples for a simple 1-component system are: * Compressibility (or its inverse, the bulk modulus) :* Isothermal compressibility ::\kappa_T=-\frac\left(\frac\right)_T \quad = -\frac\,\frac :* Adiabatic compressibility ::\kappa_S=-\frac\left(\frac\right)_S \quad = -\frac\,\frac * Specific heat (Note - the extensive analog is the heat capacity) :* Specific heat at constant pressure ::c_P=\frac\left(\frac\right)_P \quad = -\frac\,\frac :* Specific heat at constant volume ::c_V=\frac\left(\frac\right)_V \quad = -\frac\,\frac * Coefficient of thermal expansion ::\alpha=\frac\left(\frac\right)_P \quad = \frac\,\frac where ''P'' is pressure, ''V'' is volume, ''T'' is temperature, ''S'' is entropy, and ''N'' is the number of particles. For a single comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melting Point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa. When considered as the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization point. Because of the ability of substances to supercool, the freezing point can easily appear to be below its actual value. When the "characteristic freezing point" of a substance is determined, in fact, the actual methodology is almost always "the principle of observing the disappearance rather than the formation of ice, that is, the melting point." Examples For most substances, melting and freezing points are approximately equal. For example, the melting point ''and'' freezing point of mercury is . How ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linia Dilato (north Poland)
{{disambig ...
Here are places that have the name Linia: In Africa *Linia, Chad In Europe * Linia, Corfu, Greece * Linia, a village in Budeşti Commune, Vâlcea County, Romania * Linia, a village in Grădiştea Commune, Vâlcea County, Romania See also *Linea *Linia, Pomeranian Voivodeship Linia ( csb, Lëniô; formerly german: Linde) is a village in Wejherowo County, Pomeranian Voivodeship, in northern Poland. It is the seat of the gmina (administrative district) called Gmina Linia. It lies approximately south-west of Wejherow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid
Solid is one of the State of matter#Four fundamental states, four fundamental states of matter (the others being liquid, gas, and Plasma (physics), plasma). The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural rigidity and resistance to a force applied to the surface. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire available volume like a gas. The atoms in a solid are bound to each other, either in a regular geometric lattice (crystal, crystalline solids, which include metals and ordinary ice), or irregularly (an amorphous solid such as common window glass). Solids cannot be compressed with little pressure whereas gases can be compressed with little pressure because the molecules in a gas are loosely packed. The branch of physics that deals with solids is called solid-state physics, and is the main branch of condens ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Young's Modulus
Young's modulus E, the Young modulus, or the modulus of elasticity in tension or compression (i.e., negative tension), is a mechanical property that measures the tensile or compressive stiffness of a solid material when the force is applied lengthwise. It quantifies the relationship between tensile/compressive stress \sigma (force per unit area) and axial strain \varepsilon (proportional deformation) in the linear elastic region of a material and is determined using the formula: E = \frac Young's moduli are typically so large that they are expressed not in pascals but in gigapascals (GPa). Example: * Silly Putty (increasing pressure: length increases quickly, meaning tiny E) * Aluminum (increasing pressure: length increases slowly, meaning high E) Higher Young's modulus corresponds to greater (lengthwise) stiffness. Although Young's modulus is named after the 19th-century British scientist Thomas Young, the concept was developed in 1727 by Leonhard Euler. The first experime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
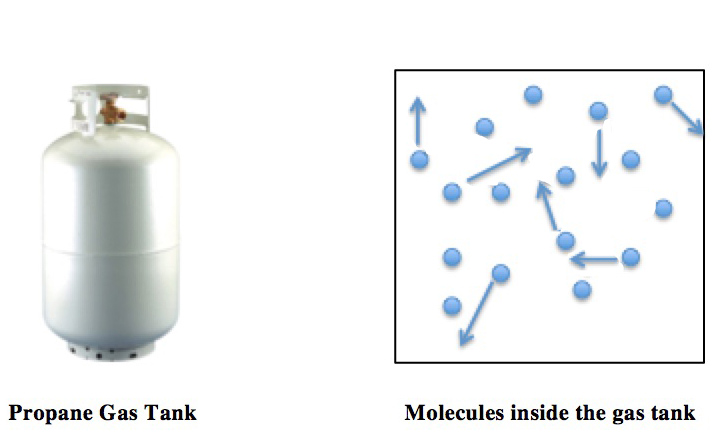
Ideal Gas Law
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stated by Benoît Paul Émile Clapeyron in 1834 as a combination of the empirical Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. The ideal gas law is often written in an empirical form: pV = nRT where p, V and T are the pressure, volume and temperature; n is the amount of substance; and R is the ideal gas constant. It can also be derived from the microscopic kinetic theory, as was achieved (apparently independently) by August Krönig in 1856 and Rudolf Clausius in 1857. Equation The state of an amount of gas is determined by its pressure, volume, and temperature. The modern form of the equation relates these simply in two main forms. The temperature used in the equation of state is an absolute temperature: the appropria ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotropy
Isotropy is uniformity in all orientations; it is derived . Precise definitions depend on the subject area. Exceptions, or inequalities, are frequently indicated by the prefix ' or ', hence ''anisotropy''. ''Anisotropy'' is also used to describe situations where properties vary systematically, dependent on direction. Isotropic radiation has the same intensity regardless of the direction of measurement, and an isotropic field exerts the same action regardless of how the test particle is oriented. Mathematics Within mathematics, ''isotropy'' has a few different meanings: ; Isotropic manifolds: A manifold is isotropic if the geometry on the manifold is the same regardless of direction. A similar concept is homogeneity. ; Isotropic quadratic form: A quadratic form ''q'' is said to be isotropic if there is a non-zero vector ''v'' such that ; such a ''v'' is an isotropic vector or null vector. In complex geometry, a line through the origin in the direction of an isotropic vector is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
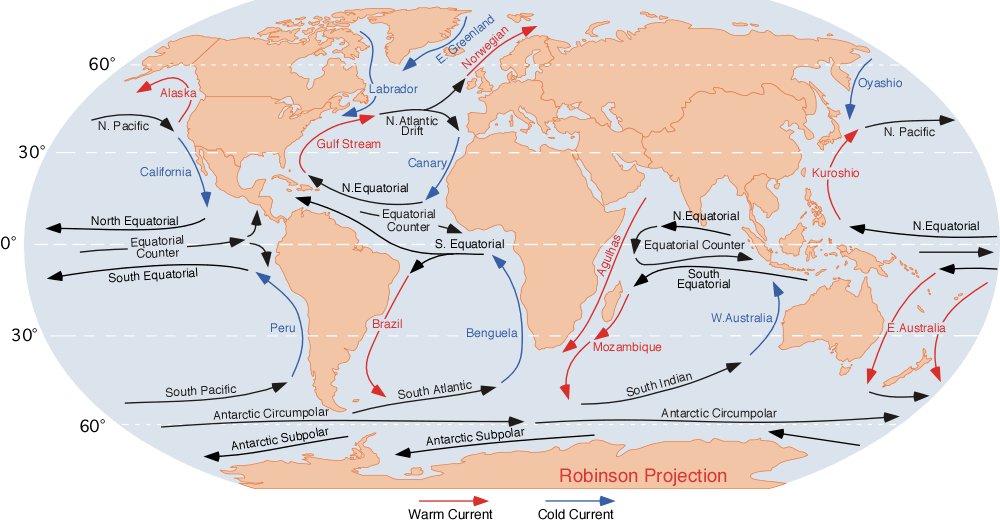
Ocean Currents
An ocean current is a continuous, directed movement of sea water generated by a number of forces acting upon the water, including wind, the Coriolis effect, breaking waves, cabbeling, and temperature and salinity differences. Depth contours, shoreline configurations, and interactions with other currents influence a current's direction and strength. Ocean currents are primarily horizontal water movements. An ocean current flows for great distances and together they create the global conveyor belt, which plays a dominant role in determining the climate of many of Earth’s regions. More specifically, ocean currents influence the temperature of the regions through which they travel. For example, warm currents traveling along more temperate coasts increase the temperature of the area by warming the sea breezes that blow over them. Perhaps the most striking example is the Gulf Stream, which makes northwest Europe much more temperate for its high latitude compared to other areas at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wind
Wind is the natural movement of air or other gases relative to a planet's surface. Winds occur on a range of scales, from thunderstorm flows lasting tens of minutes, to local breezes generated by heating of land surfaces and lasting a few hours, to global winds resulting from the difference in absorption of solar energy between the climate zones on Earth. The two main causes of large-scale atmospheric circulation are the differential heating between the equator and the poles, and the rotation of the planet (Coriolis effect). Within the tropics and subtropics, thermal low circulations over terrain and high plateaus can drive monsoon circulations. In coastal areas the sea breeze/land breeze cycle can define local winds; in areas that have variable terrain, mountain and valley breezes can prevail. Winds are commonly classified by their spatial scale, their speed and direction, the forces that cause them, the regions in which they occur, and their effect. Winds have various asp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
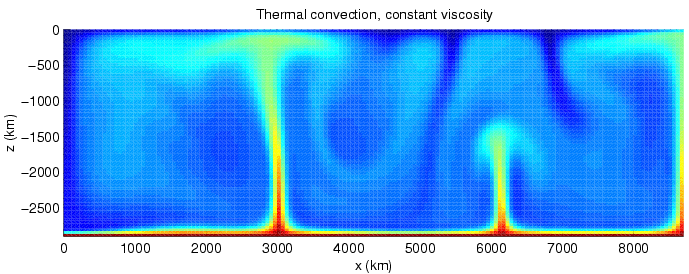
Convection
Convection is single or multiphase fluid flow that occurs spontaneously due to the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity (see buoyancy). When the cause of the convection is unspecified, convection due to the effects of thermal expansion and buoyancy can be assumed. Convection may also take place in soft solids or mixtures where particles can flow. Convective flow may be transient (such as when a multiphase mixture of oil and water separates) or steady state (see Convection cell). The convection may be due to gravitational, electromagnetic or fictitious body forces. Heat transfer by natural convection plays a role in the structure of Earth's atmosphere, its oceans, and its mantle. Discrete convective cells in the atmosphere can be identified by clouds, with stronger convection resulting in thunderstorms. Natural convection also plays a role in stellar physics. Convection is often categorised or d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
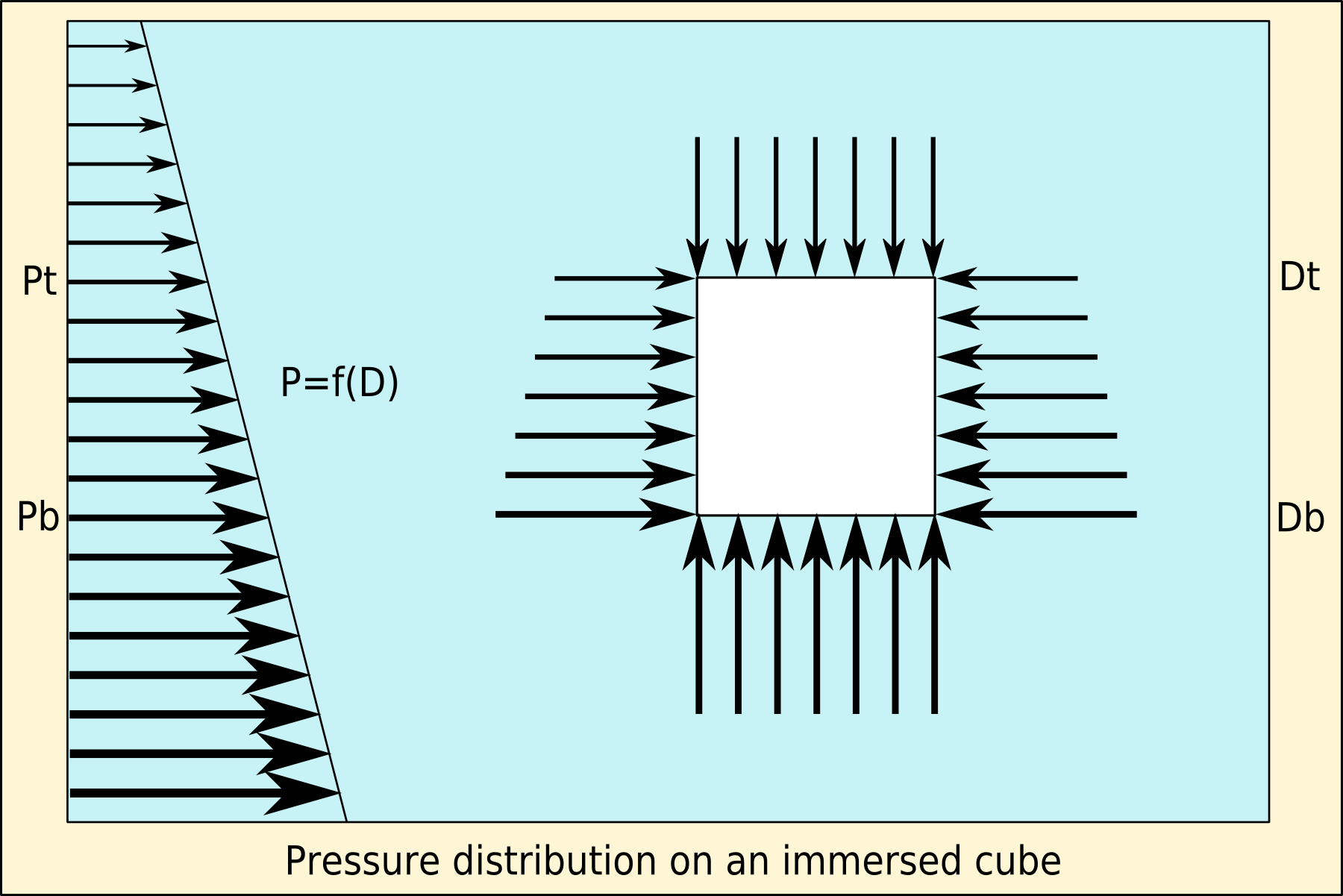
Buoyancy
Buoyancy (), or upthrust, is an upward force exerted by a fluid that opposes the weight of a partially or fully immersed object. In a column of fluid, pressure increases with depth as a result of the weight of the overlying fluid. Thus the pressure at the bottom of a column of fluid is greater than at the top of the column. Similarly, the pressure at the bottom of an object submerged in a fluid is greater than at the top of the object. The pressure difference results in a net upward force on the object. The magnitude of the force is proportional to the pressure difference, and (as explained by Archimedes' principle) is equivalent to the weight of the fluid that would otherwise occupy the submerged volume of the object, i.e. the displaced fluid. For this reason, an object whose average density is greater than that of the fluid in which it is submerged tends to sink. If the object is less dense than the liquid, the force can keep the object afloat. This can occur only in a no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |