|
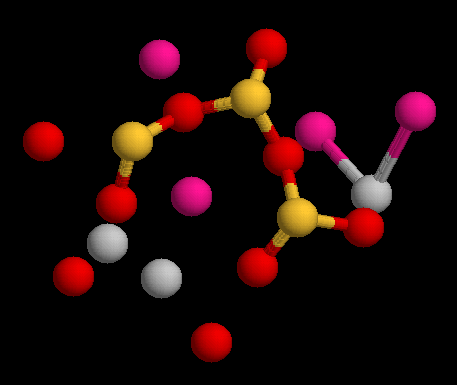
Tobermorite
Tobermorite is a calcium silicate hydrate mineral with chemical formula: Ca5Si6O16(OH)2·4H2O or Ca5Si6(O,OH)18·5H2O. Two structural varieties are distinguished: tobermorite-11 Å and tobermorite-14 Å. Tobermorite occurs in hydrated cement paste and can be found in nature as alteration mineral in metamorphosed limestone and in skarn. It has been reported from the Maqarin Area of north Jordan and in the Crestmore Quarry near Crestmore Heights, Riverside County, California. Tobermorite was first described in 1880 for an occurrence in Scotland, on the Isle of Mull, around the locality of Tobermory. Use in Roman concrete Aluminum substituted tobermorite is understood to be a key ingredient in the longevity of ancient undersea Roman concrete. The volcanic ash that Romans used for construction of sea walls contained phillipsite, and an interaction with sea water actually caused the crystalline structures in the concrete to expand and strengthen, making that material substa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jennite
Jennite is a calcium silicate hydrate mineral of general chemical formula: Ca9Si6O18(OH)6·8H2O. Jennite occurs as an alteration mineral in metamorphosed limestone and skarn. It typically occurs as vein and open space fillings as a late mineral phase. It also occurs in hydrated cement paste. A first specimen of jennite found in 1966 at the Crestmore quarries (Crestmore, Riverside County, California, US) was analysed and identified as a new mineral by Carpenter in 1966 (Carpenter, 1966). They named it in honor of its discoverer: Clarence Marvin Jenni (1896–1973) director of the Geological Museum at the University of Missouri. In contrast to the first analysis made by Carpenter, jennite does not contain appreciable amount of sodium when the Crestmore specimen was reexamined (Gard, 1977). The structure of jennite is made of three distinct modules: ribbons of edge-sharing calcium octahedra, silicate chains of wollastonite-type running along the b axis, and additional ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tobermory, Mull
Tobermory (; gd, Tobar Mhoire) is the capital of, and until 1973 the only burgh on, the Isle of Mull in the Scottish Inner Hebrides. It is located on the east coast of Mishnish, the most northerly part of the island, near the northern entrance of the Sound of Mull. The village was founded as a fishing port in 1788; its layout was based on the designs of Dumfriesshire engineer Thomas Telford. It has a current population of about 1,000. Etymology The name ''Tobermory'' is derived from the Gaelic ', meaning "Mary's well". The name refers to a well located nearby which was dedicated in ancient times to the Virgin Mary. Prehistory and archaeology Archaeological Excavations have taken place at Baliscate just outside of the town. The site was first noted by Hylda Marsh and Beverley Langhorn as part of the Scotland's Rural Past. In 2009, it was partially excavated Time Team and a further longer excavation took place in 2012 as part of a community archaeology project through the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Silicate Hydrate
Calcium silicate hydrate (or C-S-H) is the main product of the hydration of Portland cement and is primarily responsible for the strength in cement based materials (e.g. concrete). Preparation When water is added to cement, each of the compounds undergoes hydration and contributes to the final state of the concrete. Only calcium silicates contribute to the strength. Tricalcium silicate is responsible for most of the early strength (first 7 days). Dicalcium silicate, which reacts more slowly, only contributes to late strength. Calcium silicate hydrate (also shown as C-S-H) is a result of the reaction between the silicate phases of Portland cement and water. This reaction typically is expressed as: : Tricalcium silicate + water -> Calcium silicate hydrate + Calcium hydroxide + heat The stoichiometry of C-S-H in cement paste is variable and the state of chemically and physically bound water in its structure is not transparent, which is why "-" is used between C, S, and H. Synthet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Roman Concrete
Roman concrete, also called , is a material that was used in construction in ancient Rome. Roman concrete was based on a hydraulic-setting cement. It is durable due to its incorporation of pozzolanic ash, which prevents cracks from spreading. By the middle of the 1st century the material was used frequently, often brick-faced, although variations in aggregate allowed different arrangements of materials. Further innovative developments in the material, called the concrete revolution, contributed to structurally complicated forms, such as the Pantheon dome, the world's largest and oldest unreinforced concrete dome. Roman concrete was normally faced with stone or brick, and interiors might be further decorated by stucco, fresco paintings, or thin slabs of fancy colored marbles. Made up of aggregate and a two-part cementitious system it differs significantly from modern concrete. The aggregates were typically far larger than in modern concrete as well, often amounting to rubble, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicate Mineral
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust. In mineralogy, silica (silicon dioxide, ) is usually considered a silicate mineral. Silica is found in nature as the mineral quartz, and its polymorphs. On Earth, a wide variety of silicate minerals occur in an even wider range of combinations as a result of the processes that have been forming and re-working the crust for billions of years. These processes include partial melting, crystallization, fractionation, metamorphism, weathering, and diagenesis. Living organisms also contribute to this geologic cycle. For example, a type of plankton known as diatoms construct their exoskeletons ("frustules") from silica extracted from seawater. The frustules of dead diatoms are a major constituent of deep ocean sediment, and of diatomaceous earth. General structure A silicate mineral is generally ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geology Of Riverside County, California
Geology () is a branch of natural science concerned with Earth and other astronomical objects, the features or rocks of which it is composed, and the processes by which they change over time. Modern geology significantly overlaps all other Earth sciences, including hydrology, and so is treated as one major aspect of integrated Earth system science and planetary science. Geology describes the structure of the Earth on and beneath its surface, and the processes that have shaped that structure. It also provides tools to determine the relative and absolute ages of rocks found in a given location, and also to describe the histories of those rocks. By combining these tools, geologists are able to chronicle the geological history of the Earth as a whole, and also to demonstrate the age of the Earth. Geology provides the primary evidence for plate tectonics, the evolutionary history of life, and the Earth's past climates. Geologists broadly study the properties and processes of Ear ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crestmore Heights, California
Crestmore Heights is a former census-designated place in Riverside County, California, now part of the city of Jurupa Valley, California. Crestmore Heights sits at an elevation of . The 2010 United States census reported Crestmore Heights's population was 384. Geography The community is located on the south side of Fontana, north of downtown Riverside, California, and west of the Santa Ana River. According to the United States Census Bureau, the CDP covers an area of 0.3 square miles (0.7 km2), all of it land. Crestmore Quarry The former and large Crestmore Quarry is on the east side of the hill. The Riverside Portland Cement Company had its plant here, and used both the limestone and the underlying granodiorite for its manufacture of cement. Mineral specimens were also collected from the mine. As of March 2015, the mining operation of the former TXI cement manufacturing facility has stopped. As the shut down of the operations of this historic facility progressed, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cement
A cement is a binder, a chemical substance used for construction that sets, hardens, and adheres to other materials to bind them together. Cement is seldom used on its own, but rather to bind sand and gravel (aggregate) together. Cement mixed with fine aggregate produces mortar for masonry, or with sand and gravel, produces concrete. Concrete is the most widely used material in existence and is behind only water as the planet's most-consumed resource. Cements used in construction are usually inorganic, often lime or calcium silicate based, which can be characterized as hydraulic or the less common non-hydraulic, depending on the ability of the cement to set in the presence of water (see hydraulic and non-hydraulic lime plaster). Hydraulic cements (e.g., Portland cement) set and become adhesive through a chemical reaction between the dry ingredients and water. The chemical reaction results in mineral hydrates that are not very water-soluble and so are quite durable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Minerals
Calcium is a chemical element with the Symbol (chemistry), symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin language, Latin ''calx'' "lime (material), lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are wid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Compounds
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' " lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharmace ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |