|
Thiol-ene Reaction
In organosulfur chemistry, the thiol-ene reaction (also alkene hydrothiolation) is an organic reaction between a thiol () and an alkene () to form a thioether (). This reaction was first reported in 1905, but it gained prominence in the late 1990s and early 2000s for its feasibility and wide range of applications. This reaction is accepted as a click chemistry reaction given the reactions’ high yield, stereoselectivity, high rate, and thermodynamic driving force. The reaction results in an anti-Markovnikov addition of a thiol compound to an alkene. Given the stereoselectivity, high rate and yields, this synthetically useful reaction may underpin future applications in material and biomedical sciences. Mechanisms Radical addition Thiol-ene additions are known to proceed through two mechanisms: free-radical additions and catalyzed Michael additions. Free-radical additions can be initiated by light, heat or radical initiators, which form a thiyl radical species. The r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosulfur Chemistry
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries. Sulfur shares the chalcogen group with oxygen, selenium, and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium, and carbon–tellurium compounds. A classical chemical test for the detection of sulfur compoun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
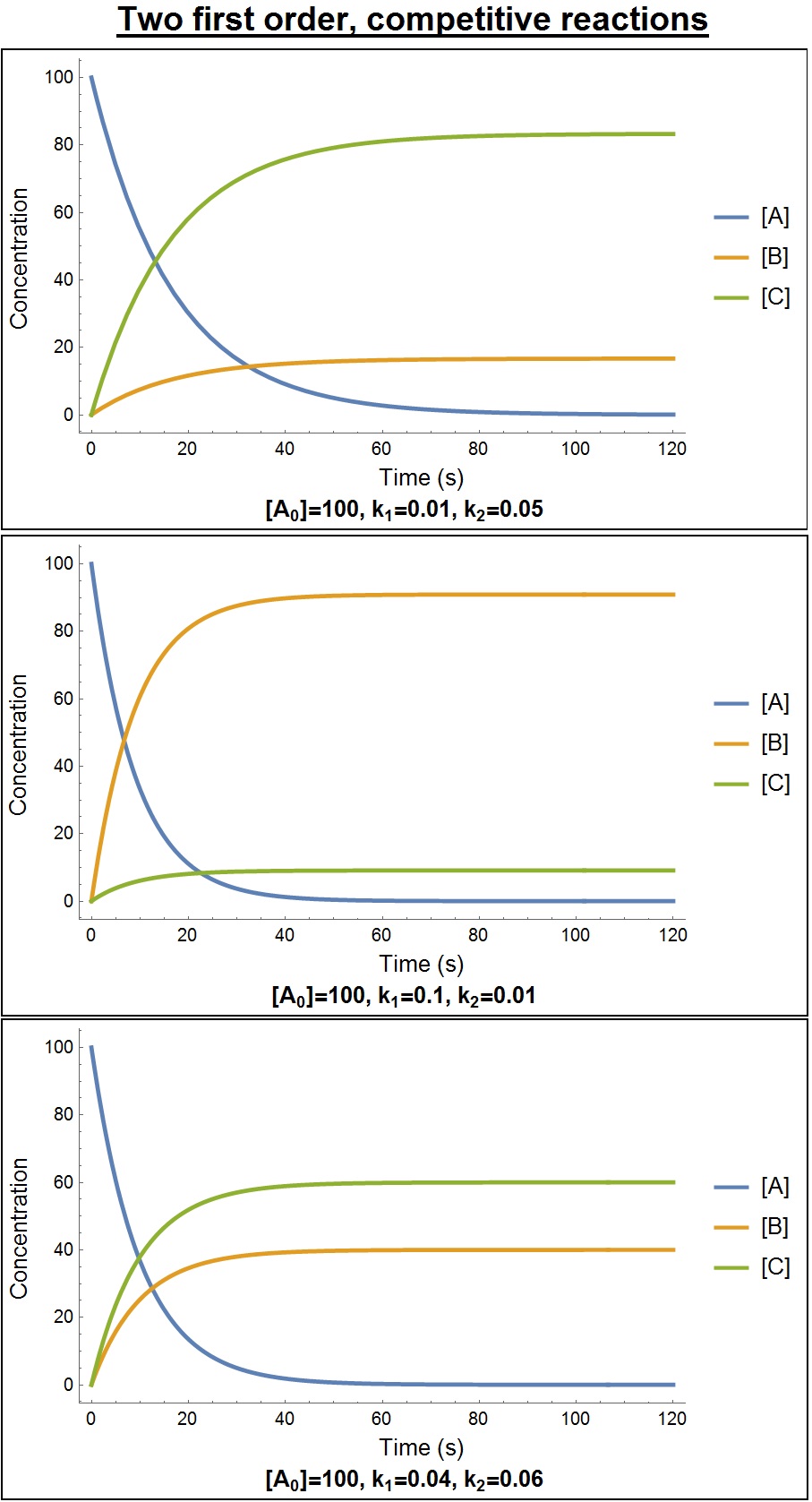
Chemical Kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction. History In 1864, Peter Waage and Cato Guldberg pioneered the development of chemical kinetics by formulating the law of mass action, which states that the speed of a chemical reaction is proportional to the quantity of the reacting substances.C.M. Guldberg and P. Waage,"Studies Concerning Affinity" ''Forhandlinger i Videnskabs-Selskabet i Christiania'' (1864), 3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiol-yne Reaction
The thiol-yne reaction (also known as alkyne hydrothiolation) is an organic reaction between a thiol and an alkyne. The reaction product is an alkenyl sulfide. The reaction was first reported in 1949 with thioacetic acid as reagent and rediscovered in 2009. It is used in click chemistry and in polymerization, especially with dendrimers. This addition reaction is typically facilitated by a radical initiator or UV irradiation and proceeds through a sulfanyl radical species. With monoaddition a mixture of (''E''/''Z'')-alkenes form. The mode of addition is anti-Markovnikov. The radical intermediate can engage in secondary reactions such as cyclisation. With diaddition the 1,2-disulfide or the 1,1- dithioacetal forms. Reported catalysts for radical additions are triethylborane, indium(III) bromide and AIBN. The reaction is also reported to be catalysed by cationic rhodium and iridium complexes, by thorium and uranium complexes, by rhodium complexes, by caesium carbonate and by g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gaussian Distribution
In statistics, a normal distribution or Gaussian distribution is a type of continuous probability distribution for a real-valued random variable. The general form of its probability density function is : f(x) = \frac e^ The parameter \mu is the mean or expectation of the distribution (and also its median and mode), while the parameter \sigma is its standard deviation. The variance of the distribution is \sigma^2. A random variable with a Gaussian distribution is said to be normally distributed, and is called a normal deviate. Normal distributions are important in statistics and are often used in the natural and social sciences to represent real-valued random variables whose distributions are not known. Their importance is partly due to the central limit theorem. It states that, under some conditions, the average of many samples (observations) of a random variable with finite mean and variance is itself a random variable—whose distribution converges to a normal distr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exponential Decay
A quantity is subject to exponential decay if it decreases at a rate proportional to its current value. Symbolically, this process can be expressed by the following differential equation, where is the quantity and (lambda) is a positive rate called the exponential decay constant, disintegration constant, rate constant, or transformation constant: :\frac = -\lambda N. The solution to this equation (see derivation below) is: :N(t) = N_0 e^, where is the quantity at time , is the initial quantity, that is, the quantity at time . Measuring rates of decay Mean lifetime If the decaying quantity, ''N''(''t''), is the number of discrete elements in a certain set, it is possible to compute the average length of time that an element remains in the set. This is called the mean lifetime (or simply the lifetime), where the exponential time constant, \tau, relates to the decay rate constant, λ, in the following way: :\tau = \frac. The mean lifetime can be looked at a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rate Equation
In chemistry, the rate law or rate equation for a reaction is an equation that links the initial or forward reaction rate with the concentrations or pressures of the reactants and constant parameters (normally rate coefficients and partial reaction orders). For many reactions, the initial rate is given by a power law such as :v_0\; =\; k mathrmx mathrmy where and express the concentration of the species and usually in moles per liter ( molarity, ). The exponents and are the partial ''orders of reaction'' for and and the ''overall'' reaction order is the sum of the exponents. These are often positive integers, but they may also be zero, fractional, or negative. The constant is the reaction rate constant or ''rate coefficient'' of the reaction. Its value may depend on conditions such as temperature, ionic strength, surface area of an adsorbent, or light irradiation. If the reaction goes to completion, the rate equation for the reaction rate v\; =\; k cex cey applies ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rate Equation
In chemistry, the rate law or rate equation for a reaction is an equation that links the initial or forward reaction rate with the concentrations or pressures of the reactants and constant parameters (normally rate coefficients and partial reaction orders). For many reactions, the initial rate is given by a power law such as :v_0\; =\; k mathrmx mathrmy where and express the concentration of the species and usually in moles per liter ( molarity, ). The exponents and are the partial ''orders of reaction'' for and and the ''overall'' reaction order is the sum of the exponents. These are often positive integers, but they may also be zero, fractional, or negative. The constant is the reaction rate constant or ''rate coefficient'' of the reaction. Its value may depend on conditions such as temperature, ionic strength, surface area of an adsorbent, or light irradiation. If the reaction goes to completion, the rate equation for the reaction rate v\; =\; k cex cey applies ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rate-determining Step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the corresponding rate equation (for comparison with the experimental rate law) is often simplified by using this approximation of the rate-determining step. In principle, the time evolution of the reactant and product concentrations can be determined from the set of simultaneous rate equations for the individual steps of the mechanism, one for each step. However, the analytical solution of these differential equations is not always easy, and in some cases numerical integration may even be required. The hypothesis of a single rate-determining step can greatly simplify the mathematics. In the simplest case the initial step is the slowest, and the overall rate is just the rate of the first step. Also, the rate equations for mechanisms with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene. Although butadiene breaks down quickly in the atmosphere, it is nevertheless found in ambient air in urban and suburban areas as a consequence of its constant emission from motor vehicles. The name butadiene can also refer to the isomer, 1,2-butadiene, which is a cumulated diene with structure H2C=C=CH−CH3. This allene has no industrial significance. History In 1863, the French chemist E. Caventou isolated butadiene from the pyrolysis of amyl alcohol. This hydrocarbon was identified as butadiene in 1886, after Henry Edward Armstrong isolated it from among the pyrolysis products of petroleum. In 1910, the Russian chemist Sergei Lebedev polymerized butadiene and obtained a materi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |