|
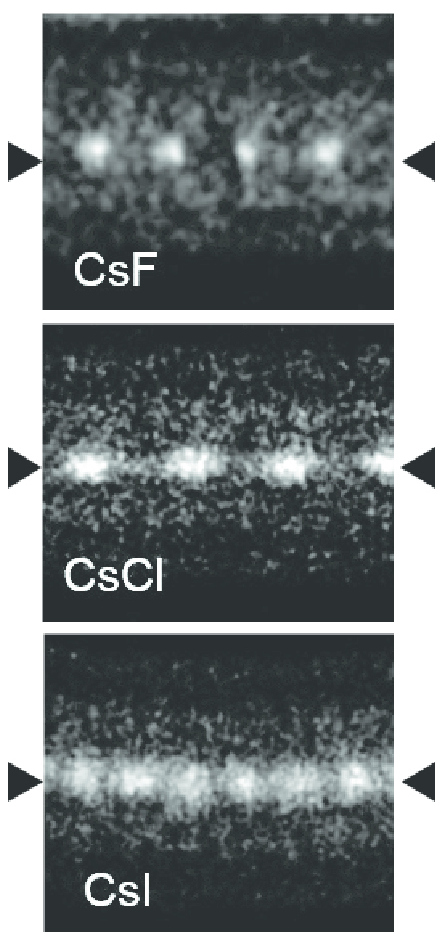
Thallium(I) Iodide
Thallium(I) iodide is a chemical compound with the formula TlI. It is unusual in being one of the few water-insoluble metal iodides, along with AgI, CuI, SnI2, SnI4, PbI2 and HgI2. Chemistry TlI can be formed in aqueous solution by metathesis of any soluble thallium salt with iodide ion. It is also formed as a by-product in thallium-promoted iodination of phenols with thallium(I) acetate. Attempts to oxidise TlI to thallium(III) iodide fail, since oxidation produces thallium(I) triiodide, . Physical properties The room temperature form of TlI is yellow and has an orthorhombic structure which can be considered to be a distorted NaCl structure. The distorted structure is believed to be caused by favourable thallium-thallium interactions, the closest Tl-Tl distance is 383 pm. At 175 °C the yellow form transforms to a red CsCl form. This phase transition is accompanied by about two orders of magnitude jump in electrical conductivity. The CsI structure can be stabilized ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the synthesis of organic compounds, and as a fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world production of ethanol was , coming mostly from Brazil and the U.S. Etymology ''Ethanol'' is the systematic name defined by the Interna ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule. Phenols are both synthesized industrially and produced by plants and microorganisms. Properties Acidity Phenols are more acidic than typical alcohols. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12). Deprotonation of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides (aryloxides according to the IUPAC Gold Book). Condensation with aldehydes and ketones Phenols are susceptible to Electrophilic aromatic substitutions. Condensation with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Russia
Russia (, , ), or the Russian Federation, is a transcontinental country spanning Eastern Europe and Northern Asia. It is the largest country in the world, with its internationally recognised territory covering , and encompassing one-eighth of Earth's inhabitable landmass. Russia extends across eleven time zones and shares land boundaries with fourteen countries, more than any other country but China. It is the world's ninth-most populous country and Europe's most populous country, with a population of 146 million people. The country's capital and largest city is Moscow, the largest city entirely within Europe. Saint Petersburg is Russia's cultural centre and second-largest city. Other major urban areas include Novosibirsk, Yekaterinburg, Nizhny Novgorod, and Kazan. The East Slavs emerged as a recognisable group in Europe between the 3rd and 8th centuries CE. Kievan Rus' arose as a state in the 9th century, and in 988, it adopted Orthodox Christianity from the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Avachinsky
Avachinsky (also known as Avacha or Avacha Volcano or Avachinskaya Sopka) (russian: Авачинская сопка, Авача) is an active stratovolcano in Russia. It is situated on the Kamchatka Peninsula in the Russian Far East. Avachinsky lies within sight of Petropavlovsk-Kamchatsky, the capital of Kamchatka Krai. Together with neighboring Koryaksky volcano, it has been designated a Decade Volcano, worthy of particular study in light of its history of explosive eruptions and proximity to populated areas. Avachinsky's last eruption occurred in 2008. This eruption was tiny compared to the volcano's major Volcanic Explosivity Index 4 eruption in 1945. Geological history Avachinsky lies on the Pacific Ring of Fire, at a point where the Pacific Plate is sliding underneath the Eurasian Plate at a rate of about /year. A wedge of mantle material lying between the subducting Pacific Plate and the overlying Eurasian Plate is the source of dynamic volcanism over the whole Kamchatka ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fumarole
A fumarole (or fumerole) is a vent in the surface of the Earth or other rocky planet from which hot volcanic gases and vapors are emitted, without any accompanying liquids or solids. Fumaroles are characteristic of the late stages of volcanic activity, but fumarole activity can also precede a volcanic eruption and has been used for eruption prediction. Most fumaroles die down within a few days or weeks of the end of an eruption, but a few are persistent, lasting for decades or longer. An area containing fumaroles is known as a fumarole field. The predominant vapor emitted by fumaroles is steam, formed by the circulation of groundwater through heated rock. This is typically accompanied by volcanic gases given off by magma cooling deep below the surface. These volcanic gases include sulfur compounds, such as various sulfur oxides and hydrogen sulfide, and sometimes hydrogen chloride, hydrogen fluoride, and other gases. A fumarole that emits significant sulfur compounds is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mascagnite
Mascagnite is a rare ammonium sulfate mineral ( N H4)2 S O4. It crystallizes in the orthorhombic system typically forming as stalactitic masses exhibiting good cleavage. It is soft (not higher than 2.5 on the Mohs Scale) and water-soluble. Optical properties are variable; the purest form is transparent and colorless, but opaque gray or yellow deposits are also known. It occurs in fumaroles, as at Mount Vesuvius Mount Vesuvius ( ; it, Vesuvio ; nap, 'O Vesuvio , also or ; la, Vesuvius , also , or ) is a somma- stratovolcano located on the Gulf of Naples in Campania, Italy, about east of Naples and a short distance from the shore. It is one of ... and associated with coal seam fires. It was named for Italian anatomist Paolo Mascagni (1752–1815) who first described the mineral. References Ammonium minerals Sulfate minerals Orthorhombic minerals Minerals in space group 62 Mount Vesuvius {{sulfate-mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scintillator
A scintillator is a material that exhibits scintillation, the property of luminescence, when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate (i.e. re-emit the absorbed energy in the form of light). Sometimes, the excited state is metastable, so the relaxation back down from the excited state to lower states is delayed (necessitating anywhere from a few nanoseconds to hours depending on the material). The process then corresponds to one of two phenomena: delayed fluorescence or phosphorescence. The correspondence depends on the type of transition and hence the wavelength of the emitted optical photon. Principle of operation A scintillation detector or scintillation counter is obtained when a scintillator is coupled to an electronic light sensor such as a photomultiplier tube (PMT), photodiode, or silicon photomultiplier. PMTs absorb the light emitted by the scintillator and re-emit it in the form o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium Iodide
Caesium iodide or cesium iodide (chemical formula CsI) is the ionic compound of caesium and iodine. It is often used as the input phosphor of an X-ray image intensifier tube found in fluoroscopy equipment. Caesium iodide photocathodes are highly efficient at extreme ultraviolet wavelengths. Synthesis and structure Bulk caesium iodide crystals have the cubic CsCl crystal structure, but the structure type of nanometer-thin CsI films depends on the substrate material – it is CsCl for mica and NaCl for LiF, NaBr and NaCl substrates. Caesium iodide atomic chains can be grown inside double-wall carbon nanotubes. In such chains I atoms appear brighter than Cs atoms in electron micrographs despite having a smaller mass. This difference was explained by the charge difference between Cs atoms (positive), inner nanotube walls (negative) and I atoms (negative). As a result, Cs atoms are attracted to the walls and vibrate more strongly than I atoms, which are pushed toward the nanotube a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Iodide
Sodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anions (I−) in a crystal lattice. It is used mainly as a nutritional supplement and in organic chemistry. It is produced industrially as the salt formed when acidic iodides react with sodium hydroxide. It is a chaotropic salt. Uses Food supplement Sodium iodide, as well as potassium iodide, is commonly used to treat and prevent iodine deficiency. Iodized table salt contains 10 ppm iodide. Organic synthesis Sodium iodide is used for conversion of alkyl chlorides into alkyl iodides. This method, the Finkelstein reaction, relies on the insolubility of sodium chloride in acetone to drive the reaction: ::R–Cl + NaI → R–I + NaCl Nuclear medicine Some radioactive iodide salts of sodium, including Na 125I and Na 131I, have radio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rocksalt
Halite (), commonly known as rock salt, is a type of salt, the mineral (natural) form of sodium chloride ( Na Cl). Halite forms isometric crystals. The mineral is typically colorless or white, but may also be light blue, dark blue, purple, pink, red, orange, yellow or gray depending on inclusion of other materials, impurities, and structural or isotopic abnormalities in the crystals. It commonly occurs with other evaporite deposit minerals such as several of the sulfates, halides, and borates. The name ''halite'' is derived from the Ancient Greek word for "salt", ἅλς (''háls''). Occurrence Halite dominantly occurs within sedimentary rocks where it has formed from the evaporation of seawater or salty lake water. Vast beds of sedimentary evaporite minerals, including halite, can result from the drying up of enclosed lakes and restricted seas. Such salt beds may be hundreds of meters thick and underlie broad areas. Halite occurs at the surface today in playas in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium Chloride
Caesium chloride or cesium chloride is the inorganic compound with the formula Cs Cl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each caesium ion is coordinated by 8 chloride ions. Caesium chloride dissolves in water. CsCl changes to NaCl structure on heating. Caesium chloride occurs naturally as impurities in carnallite (up to 0.002%), sylvite and kainite. Less than 20 tonnes of CsCl is produced annually worldwide, mostly from a caesium-bearing mineral pollucite. Caesium chloride is widely used medicine structure in isopycnic centrifugation for separating various types of DNA. It is a reagent in analytical chemistry, where it is used to identify ions by the color and morphology of the precipitate. When enriched in radioisotopes, such as 137CsCl or 131CsCl, caesium chloride is used in nuclear medicine applications such as treatment of cancer and diagnosis of myocar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Journal Of Inorganic Chemistry
The ''European Journal of Inorganic Chemistry'' is a weekly peer-reviewed scientific journal covering inorganic, organometallic, bioinorganic, and solid-state chemistry. It is published by Wiley-VCH on behalf of Chemistry Europe. The journal, along with the '' European Journal of Organic Chemistry'', was established in 1998 as the result of a merger of '' Chemische Berichte/Recueil,'' '' Bulletin de la Société Chimique de France,'' '' Bulletin des Sociétés Chimiques Belges,'' '' Gazzetta Chimica Italiana,'' '' Anales de Química,'' ''Chimika Chronika,'' ''Revista Portuguesa de Química, and'' ''ACH-Models in Chemistry.'' According to the ''Journal Citation Reports'', the journal has a 2021 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as ... of 2.551. See also * L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)