|
Tetramethylammonium Fluoride
Tetramethylammonium fluoride is the quaternary ammonium salt with the formula (CH3)4NF. This hygroscopic white solid is a source of “naked fluoride", that is fluoride ions not connected to a metal atom in a complex. Most other soluble salts of fluoride are in fact bifluorides, HF2–. Historically, there has been two main approaches to prepare TMAF: (i) Via neutralization of tetramethylammonium hydroxide (TMAOH) with HF, and (ii) through the metathesis reaction of different ammonium salts with inorganic sources of fluoride, such as KF or CsF. Due to the high basicity of the fluoride anion, the salt reacts slowly with acetonitrile, inducing its dimerization to CH3C(NH2)=CHCN, which co-crystallizes. Related salts * Tetramethylphosphonium fluoride (CH3)4PF forms stable solutions in acetonitrile. It is prepared by reaction of the ylide with potassium bifluoride: :(CH3)3P=CH2 + KHF2 → (CH3)4PF + KF :In the gas phase, tetramethylphosphonium fluoride exists as the phosphor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quaternary Ammonium Salt
In chemistry, quaternary ammonium cations, also known as quats, are positively charged polyatomic ions of the structure , R being an alkyl group or an aryl group. Unlike the ammonium ion () and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds (called quaternary amines in oilfield parlance) are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule. Quats are used in consumer applications including as antimicrobials (such as detergents and disinfectants), fabric softeners, and hair conditioners. As an antimicrobial, they are able to inactivate enveloped viruses (such as SARS-CoV-2). Quats tend to be gentler on surfaces than bleach-based disinfectants, and are generally fabric-safe. Synthesis Quaternary ammoniu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
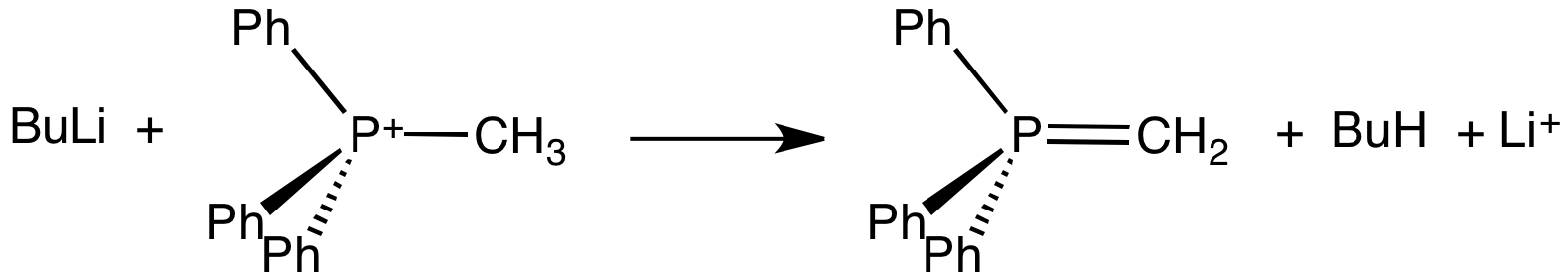
Phosphorane
A phosphorane (IUPAC name: λ5-phosphane) is a functional group in organophosphorus chemistry with pentavalent phosphorus. It has the general formula PR5. The parent hydride compound is the hypothetical molecule PH5. The derivative pentaphenylphosphorane (Ph5P) is stable. Phosphoranes adopt a trigonal bipyramidal molecular geometry with the two apical bonds longer than the three equatorial bonds. Hypervalent bonding is described by inclusion of non-bonding MOs, as also invoked for the closely related molecule phosphorus pentafluoride.G. L. Miessler and D. A. Tarr “Inorganic Chemistry” 3rd Ed, Pearson/Prentice Hall publisher, . Phosphoranes of the type R3P=CR2 are more common and more important. Phosphoranes are also considered to be one of the resonance structures of ylides, these compounds feature a tetrahedral phosphorus center including a phosphorus–carbon double bond. These compounds are used as reagents in the Wittig reaction The Wittig reaction or Wittig ole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorides
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin. Fluoride is the simplest fluorine anion. In terms of charge and size, the fluoride ion resembles the hydroxide ion. Fluoride ions occur on Earth in several minerals, particularly fluorite, but are present only in trace quantities in bodies of water in nature. Nomenclature Fluorides include compounds that contain ionic fluoride and those in which fluoride does not dissociate. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramethylammonium Salts
Tetramethylammonium (TMA) or (Me4N+) is the simplest quaternary ammonium cation, consisting of four methyl groups attached to a central nitrogen atom, and is isoelectronic with neopentane. It is positively charged and can only be isolated in association with a counter-ion. Common quaternary ammonium salt, salts include tetramethylammonium chloride and tetramethylammonium hydroxide. Tetramethylammonium salts are commonly used in chemical synthesis and are widely employed in Pharmacology, pharmacological research. Common nomenclature In the toxicological literature, ''naturally occurring'' tetramethylammonium (anion unspecified) is often referred to by the name "tetramine". Unfortunately, this non-systematic or "trivial" name is also used for other chemical entities, including a toxic rodenticide (Tetramethylenedisulfotetramine). Similarly, the acronym "TMA", which is frequently used for tetramethylammonium in the pharmacological literature, may also refer to the investigational drug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of The American Chemical Society
The ''Journal of the American Chemical Society'' is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ''Journal of Analytical and Applied Chemistry'' (July 1893) and the ''American Chemical Journal'' (January 1914). It covers all fields of chemistry. Since 2021, the editor-in-chief is Erick M. Carreira ( ETH Zurich). In 2014, the journal moved to a hybrid open access publishing model. Abstracting and indexing The journal is abstracted and indexed in Chemical Abstracts Service, Scopus, EBSCO databases, ProQuest databases, Index Medicus/ MEDLINE/ PubMed, and the Science Citation Index Expanded. According to the '' Journal Citation Reports'', the journal has a 2021 impact factor of 16.383. Editors-in-chief The following people are or have been editor-in-chief: * 1879–1880 – Hermann Endemann * 1880–1881 – Gideon E. Moore * 1881–1882 – Hermann ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexafluorobenzene
Hexafluorobenzene, HFB, , or perfluorobenzene is an organic, aromatic compound. In this derivative of benzene all hydrogen atoms have been replaced by fluorine atoms. The technical uses of the compound are limited, although it is recommended as a solvent in a number of photochemical reactions. In the laboratory hexafluorobenzene is used as standard in fluorine-19 NMR spectroscopy, solvent and standard in carbon-13 NMR, solvent in proton NMR, solvent when studying some parts in the infrared and solvent in ultraviolet–visible spectroscopy, as hexafluorobenzene itself hardly shows any absorbance in the UV region. Geometry of the aromatic ring Hexafluorobenzene stands somewhat aside in the perhalogenbenzenes. When counting for bond angles and distances it is possible to calculate the distance between two ortho fluorine atoms. Also the non bonding radius of the halogens is known. The following table presents the results: The conclusion of the table is hexafluorobenzene is th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrabutylammonium Fluoride
Tetra-''n''-butylammonium fluoride, commonly abbreviated to TBAF and ''n''-Bu4NF, is a quaternary ammonium salt with the chemical formula (CH3CH2CH2CH2)4N+F−. It is commercially available as the white solid trihydrate and as a solution in tetrahydrofuran. TBAF is used as a source of fluoride ion in organic solvents. Preparation and properties TBAF can be prepared by passing hydrofluoric acid through an ion-exchange resin, followed by tetrabutylammonium bromide. Upon evaporation of the water, TBAF can be collected as an oil in quantitative yield. Preparing anhydrous samples is of interest as the basicity of fluoride increases by more than 20 p''K'' units on passing from aqueous to aprotic solvent. However, heating samples of the hydrated material to 77 °C under vacuum causes decomposition to the hydrogen difluoride salt. Similarly, samples dried at 40 °C under high vacuum still contain 10-30 mol% of water and some 10% of difluoride. Instead, anhydrous TBAF has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Bifluoride
Potassium bifluoride is the inorganic compound with the formula . This colourless salt consists of the potassium cation () and the bifluoride anion (). The salt is used as an etchant for glass. Sodium bifluoride is related and is also of commercial use as an etchant as well as in cleaning products. Synthesis and reactions The salt was prepared by Edmond Frémy by treating potassium carbonate or potassium hydroxide with hydrofluoric acid: : With one more equivalent of HF, (CAS#12178-06-2, m.p. 71.7 C) is produced: : Thermal decomposition of gives hydrogen fluoride: : Applications The industrial production of fluorine entails the electrolysis of molten and . The electrolysis of was first used by Henri Moissan Ferdinand Frédéric Henri Moissan (28 September 1852 – 20 February 1907) was a French chemist and pharmacist who won the 1906 Nobel Prize in Chemistry for his work in isolating fluorine from its compounds. Moissan was one of the original me ... in 1886. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin. Fluoride is the simplest fluorine anion. In terms of charge and size, the fluoride ion resembles the hydroxide ion. Fluoride ions occur on Earth in several minerals, particularly fluorite, but are present only in trace quantities in bodies of water in nature. Nomenclature Fluorides include compounds that contain ionic fluoride and those in which fluoride does not dissociate. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ylide
An ylide or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. The result can be viewed as a structure in which two adjacent atoms are connected by both a covalent and an ionic bond; normally written X+–Y−. Ylides are thus 1,2- dipolar compounds, and a subclass of zwitterions. They appear in organic chemistry as reagents or reactive intermediates. The class name "ylide" for the compound should not be confused with the suffix "-ylide". Resonance structures Many ylides may be depicted by a multiple bond form in a resonance structure, known as the ylene form, while the actual structure lies in between both forms: : The actual bonding picture of these types of ylides is strictly zwitterionic (the structure on the right) with the strong Coulombic attraction betw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.png)