|
Tetrahydroxozincate
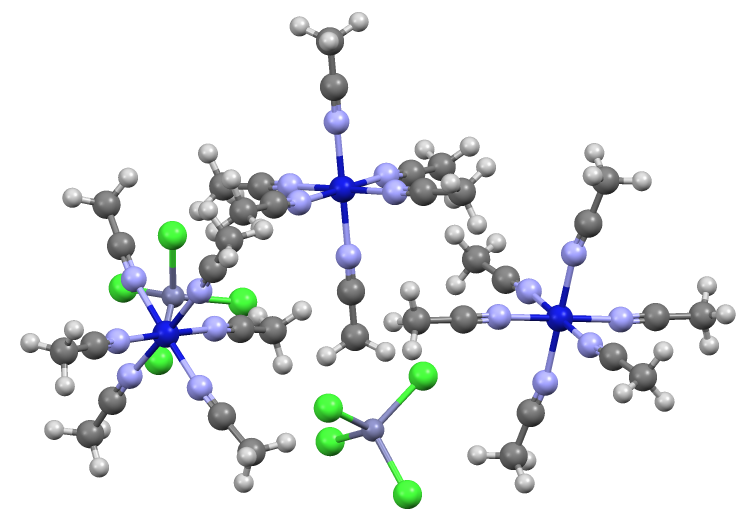
In chemistry, tetrahydroxozincate or tetrahydroxidozincate is a divalent anion (negative ion) with formula , with a central zinc atom in the +2 or (II) valence state coordinated to four hydroxide groups. It has Sp3 hybridization. It is the most common of the zincate anions, and is often called just zincate. These names are also used for the salts containing that anion, such as sodium zincate Na2Zn(OH)4 and calcium zincate CaZn(OH)4·2H2O Zincate salts can be obtained by reaction of zinc oxide (ZnO) or zinc hydroxide () and a strong base like sodium hydroxide. It is now generally accepted that the resulting solutions contain the tetrahydroxozincate ion. Earlier Raman studies had been interpreted as indicating the existence of linear ions. Related anions and salts The name "zincate" may also refer to a polymeric anion with formula approaching []''n'', which forms salts such as ·, or to mixed oxides of zinc and less electronegativity, electronegative elements, such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a Chemical reaction, reaction with other Chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Base (chemistry)
In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form Hydroxide ions OH−. These ions can react with hydrogen ions (H+ according to Arrhenius) from the dissociation of acids to form water in an acid–base reaction An acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their applica .... A base was therefore a metal hydroxide such as Sodium hydroxide, NaOH or Calcium hydroxide, Ca(OH)2. Such aqueous hydroxide solutions were also described by certain characteristic properties. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC Nomenclature Of Inorganic Chemistry 2005
Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005 is the 2005 version of ''Nomenclature of Inorganic Chemistry'' (which is informally called the Red Book). It is a collection of rules for naming inorganic compounds, as recommended by the International Union of Pure and Applied Chemistry (IUPAC). Summary The 2005 edition replaces their previous recommendations ''Nomenclature The Red Book of Inorganic Chemistry, IUPAC Recommendations 1990 (Red Book I)'', and "where appropriate" (sic) ''Nomenclature of Inorganic Chemistry II, IUPAC Recommendations 2000 (Red Book II)''. The recommendations take up over 300 pages''Nomenclature of Inorganic Chemistry IUPAC Recommendations'' 2005 ed. N. G. Connelly et al. RSC Publishing https://iupac.org/what-we-do/books/redbook/ and the full text can be downloaded from IUPAC. Corrections have been issued. Apart from a reorganisation of the content, there is a new section on organometallics and a formal element list to be used in place of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeitschrift Für Anorganische Und Allgemeine Chemie
The ''Zeitschrift für anorganische und allgemeine Chemie'' (''Journal of Inorganic and General Chemistry'') is a semimonthly peer-reviewed scientific journal covering inorganic chemistry, published by Wiley-VCH. The editors-in-chief are Thomas F. Fässler, Christian Limberg, Guodong Qian, and David Scheschkewitz. Originally the journal was published in German, but nowadays it is completely in English. Abstracting and indexing The journal is abstracted and indexed in the following databases: According to the ''Journal Citation Reports'', the journal has a 2021 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as i ... of 1.414, ranking it 40th out of 46 journals in the category "Chemistry, Inorganic & Nuclear". References External links * Chemistry journals Wiley-VCH aca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrachlorozincate
Tetrachlorozincate is an anion with the formula nCl4sup>2−. It is a counterion that is often used in conjunction with strong electrophiles. Being dianionic, tetrachlorozincate is not classified as a weakly coordinating anion. On the other hand, being dianionic, tetrachlorozincate facilitates the crystallization of many salts. It has a tetrahedral molecular geometry. A simple example is (NH4)2 nCl4 The anion is tetrahedral. Zincates are anionic zinc complexes. Related to the preparation of Lucas' reagent, tetrachlorozincates are often generated by combining hydrochloric acid Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid Acid strength is the tendency of an acid, symbol ... and zinc chloride. A related anion is n2Cl6sup>2−, in which again Zn(II) adopts a tetrahedral geometry. References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity, the more an atom or a substituent group attracts electrons. Electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's chemical polarity, which characterizes a bond along the continuous scale from covalent to ionic bonding. The loosely defined term electropositivity is the opposite of electronegativity: it characterizes an element's tendency to donate valence electrons. On the most basic level, electronegativity is determined by factors like the nuclear charge (the more protons an atom has, the more "pull" it will have on electrons) and the number and location ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Raman Studies
Raman spectroscopy () (named after Indian physicist C. V. Raman) is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman spectroscopy is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified. Raman spectroscopy relies upon inelastic scattering of photons, known as Raman scattering. A source of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range is used, although X-rays can also be used. The laser light interacts with molecular vibrations, phonons or other excitations in the system, resulting in the energy of the laser photons being shifted up or down. The shift in energy gives information about the vibrational modes in the system. Infrared spectroscopy typically yields similar yet complementary information. Typically, a sample is illuminated with a laser beam. Electrom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions . Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates . The monohydrate crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students. Sodium hydroxide is used in many industries: in the manufacture of pulp and paper, textiles, drinking water, soaps and deterge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Hydroxide
Zinc hydroxide Zn( OH)2 is an inorganic chemical compound. It also occurs naturally as 3 rare minerals: wülfingite (orthorhombic), ashoverite and sweetite (both tetragonal). Like the hydroxides of other metals, such as lead, aluminium, beryllium, tin and chromium, Zinc hydroxide (and Zinc oxide), is amphoteric. Thus it will dissolve readily in a dilute solution of a strong acid, such as HCl, and also in a solution of an alkali such as sodium hydroxide. Preparation It can be prepared by adding sodium hydroxide solution, but not in excess, to a solution of any zinc salt. A white precipitate will be seen: :Zn2+ + 2 OH− → Zn(OH)2. Zn2+ is known to form hexa-aqua ions at high water concentrations and tetra-aqua ions at low concentrations of water Sze, Yu-Keung, and Donald E. Irish. "Vibrational spectral studies of ion-ion and ion-solvent interactions. I. Zinc nitrate in water." Journal of Solution Chemistry 7.6 (1978): 395-415. and, thus, this reaction may be better writt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valence (chemistry)
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Description The combining capacity, or affinity of an atom of a given element is determined by the number of hydrogen atoms that it combines with. In methane, carbon has a valence of 4; in ammonia, nitrogen has a valence of 3; in water, oxygen has a valence of 2; and in hydrogen chloride, chlorine has a valence of 1. Chlorine, as it has a valence of one, can be substituted for hydrogen. Phosphorus has a valence of 5 in phosphorus pentachloride, . Valence diagrams of a compound represent the connectivity of the elements, with lines drawn between two elements, sometimes called bonds, representing a saturated valency for each element. The two tables below show some examples of different compounds, their valence diagrams, and the valences for each element of the compound. Modern definitions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Oxide
Zinc oxide is an inorganic compound with the formula . It is a white powder that is insoluble in water. ZnO is used as an additive in numerous materials and products including cosmetics, food supplements, rubbers, plastics, ceramics, glass, cement, lubricants, paints, ointments, adhesives, sealants, pigments, foods, batteries, ferrites, fire retardants, and first-aid tapes. Although it occurs naturally as the mineral zincite, most zinc oxide is produced synthetically. ZnO is a wide-band gap semiconductor of the II-VI semiconductor group. The native doping of the semiconductor due to oxygen vacancies or zinc interstitials is n-type. Other favorable properties include good transparency, high electron mobility, wide band gap, and strong room-temperature luminescence. Those properties make ZnO valuable for a variety of emerging applications: transparent electrodes in liquid crystal displays, energy-saving or heat-protecting windows, and electronics as thin-film transistors and lig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |