|
Tetrafluoromethane
Tetrafluoromethane, also known as carbon tetrafluoride or R-14, is the simplest perfluorocarbon ( C F4). As its IUPAC name indicates, tetrafluoromethane is the perfluorinated counterpart to the hydrocarbon methane. It can also be classified as a haloalkane or halomethane. Tetrafluoromethane is a useful refrigerant but also a potent greenhouse gas. It has a very high bond strength due to the nature of the carbon–fluorine bond. Bonding Because of the multiple carbon–fluorine bonds, and the high electronegativity of fluorine, the carbon in tetrafluoromethane has a significant positive partial charge which strengthens and shortens the four carbon–fluorine bonds by providing additional ionic character. Carbon–fluorine bonds are the strongest single bonds in organic chemistry. Additionally, they strengthen as more carbon–fluorine bonds are added to the same carbon. In the one carbon organofluorine compounds represented by molecules of fluoromethane, difluoromethane, tri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluorocarbon
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often has distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics. Nomenclature Perfluorocarbons or PFCs, are organofluorine compounds with the formula CxFy, i.e., they contain only carbon and fluorine. The terminology is not strictly followed and many fluorine-containing organic compounds are called fluorocarbons. Compounds with the prefix perfluoro- are hydrocarbons, including those with heteroatoms, wherein all C-H bonds have been replaced by C-F bonds. Fluorocarbons includes perfluoroalkanes, fluoroalkenes, fluoroalkynes, and perfluoroaromatic compounds. Perfluoroalkanes Chemical properties Perfluoroalkanes are very stable because of the strength of the carbon–fluorine bond, one of the strongest in organic chemistry. Its strength is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organofluorine Compound
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents. The carbon–fluorine bond Fluorine has several distinctive differences from all other substituents encountered in organic molecules. As a result, the physical and chemical properties of organofluorines can be distinctive in comparison to other organohalogens. # The carbon–fluorine bond is one of the strongest in organic chemistry (an average bond energy around 480 kJ/molKirsch, Peer ''Modern flu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haloalkane
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for moder ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon–fluorine Bond
The carbon–fluorine bond is a polar covalent bond between carbon and fluorine that is a component of all organofluorine compounds. It is one of the strongest single bonds in chemistry (after the B–F single bond, Si–F single bond, and H–F single bond), and relatively short, due to its partial ionic character. The bond also strengthens and shortens as more fluorines are added to the same carbon on a chemical compound. As such, fluoroalkanes like tetrafluoromethane (carbon tetrafluoride) are some of the most unreactive organic compounds. Electronegativity and bond strength The high electronegativity of fluorine (4.0 for fluorine vs. 2.5 for carbon) gives the carbon–fluorine bond a significant polarity or dipole moment. The electron density is concentrated around the fluorine, leaving the carbon relatively electron poor. This introduces ionic character to the bond through partial charges (Cδ+—Fδ−). The partial charges on the fluorine and carbon are attract ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Greenhouse Gas
A greenhouse gas (GHG or GhG) is a gas that absorbs and emits radiant energy within the thermal infrared range, causing the greenhouse effect. The primary greenhouse gases in Earth's atmosphere are water vapor (), carbon dioxide (), methane (), nitrous oxide (), and ozone (). Without greenhouse gases, the average temperature of Earth's surface would be about , rather than the present average of . The atmospheres of Venus, Mars and Titan also contain greenhouse gases. Human activities since the beginning of the Industrial Revolution (around 1750) have increased the atmospheric concentration of carbon dioxide by over 50%, from 280 ppm in 1750 to 421 ppm in 2022. The last time the atmospheric concentration of carbon dioxide was this high was over 3 million years ago. This increase has occurred despite the absorption of more than half of the emissions by various natural carbon sinks in the carbon cycle. At current greenhouse gas emission rates, temperatures co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an inorganic compound with the chemical formula . This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers, e.g. polytetrafluoroethylene (PTFE). HF is widely used in the petrochemical industry as a component of superacids. Hydrogen fluoride boils at near room temperature, much higher than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid had been known in the glass industry before then. French chemist Edmond Frémy (1814–1894) is credited with disco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coulombic
Coulomb's inverse-square law, or simply Coulomb's law, is an experimental law of physics that quantifies the amount of force between two stationary, electrically charged particles. The electric force between charged bodies at rest is conventionally called ''electrostatic force'' or Coulomb force. Although the law was known earlier, it was first published in 1785 by French physicist Charles-Augustin de Coulomb, hence the name. Coulomb's law was essential to the development of the theory of electromagnetism, maybe even its starting point, as it made it possible to discuss the quantity of electric charge in a meaningful way. The law states that the magnitude of the electrostatic force of attraction or repulsion between two point charges is directly proportional to the product of the magnitudes of charges and inversely proportional to the square of the distance between them. Coulomb studied the repulsive force between bodies having electrical charges of the same sign: Coulomb als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
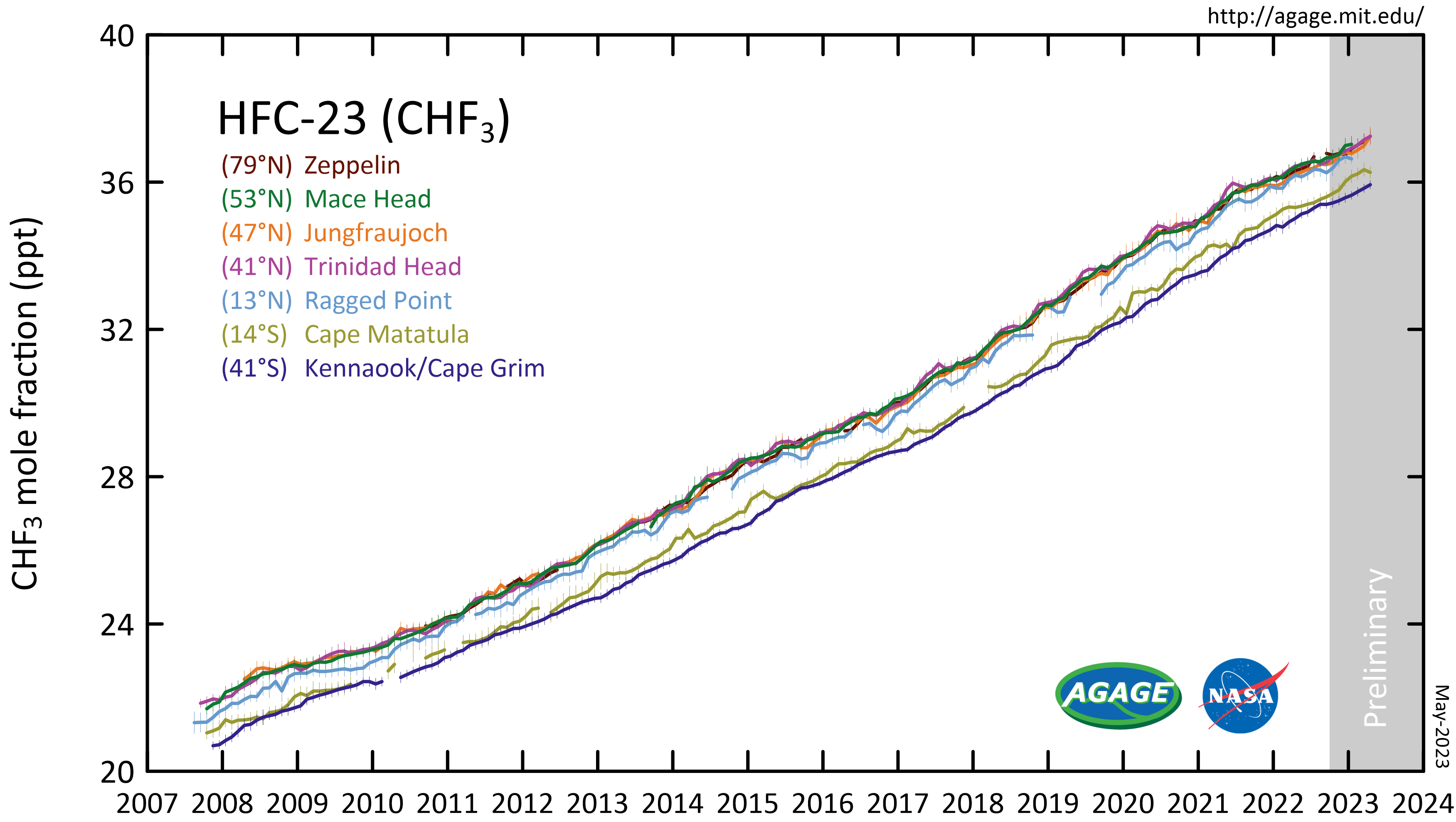
Trifluoromethane
Trifluoromethane or fluoroform is the chemical compound with the formula CHF3. It is one of the " haloforms", a class of compounds with the formula CHX3 (X = halogen) with C3v symmetry. Fluoroform is used in diverse applications in organic synthesis. It is not an ozone depleter but is a greenhouse gas. Synthesis About 20M kg/y are produced industrially as both a by-product of and precursor to the manufacture of Teflon. It is produced by reaction of chloroform with HF: :CHCl3 + 3 HF → CHF3 + 3 HCl It is also generated biologically in small amounts apparently by decarboxylation of trifluoroacetic acid. Historical Fluoroform was first obtained by Maurice Meslans in the violent reaction of iodoform with dry silver fluoride in 1894. The reaction was improved by Otto Ruff by substitution of silver fluoride by a mixture of mercury fluoride and calcium fluoride. The exchange reaction works with iodoform and bromoform, and the exchange of the first two halogen atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Difluoromethane
Difluoromethane, also called difluoromethylene, HFC-32 Methylene Fluoride or R-32, is an organic compound of the dihalogenoalkane variety. It has the formula of CH2F2. It is a colorless gas in the ambient atmosphere and is slightly soluble in the water, with a high thermal stability. Due to the low melting and boiling point, (-136.0 °C and -51.6 °C respectively) contact with this compound may result in frostbite. In the United States, the Clean Air Act Section 111 on Volatile Organic Compounds (VOC) has listed difluoromethane as an exception (since 1997) from the definition of VOC due to its low production of tropospheric ozone. Difluoromethane is commonly used in endothermic processes such as refrigeration or air conditioning. Synthesis Difluoromethane is primarily synthesized via batch processes, by the reaction of dichloromethane and hydrogen fluoride (HF), in the liquid phase using SbCl5 as a catalyst. Due to hydrogen fluoride's hazardous properties, a new synt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoromethane
Fluoromethane, also known as methyl fluoride, Freon 41, Halocarbon-41 and HFC-41, is a non-toxic, liquefiable, and flammable gas at standard temperature and pressure. It is made of carbon, hydrogen, and fluorine. The name stems from the fact that it is methane (CH4) with a fluorine atom substituted for one of the hydrogen atoms. It is used in semiconductor manufacturing processes as an etching gas in plasma etch reactors. Composition The compound is the lowest mass member of the hydrofluorocarbon (HFC) family, compounds which contain only hydrogen, fluorine, and carbon. These compounds are related to the chlorofluorocarbons (CFC), but since they do not contain chlorine, are not destructive to the ozone layer. Fluorocarbons are, however, potent greenhouse gasses, and the Kigali Amendment to the Montreal Protocol The Montreal Protocol is an international treaty A treaty is a formal, legally binding written agreement between actors in international law. It is usua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |