|
Tetraethoxymethane
Tetraethoxymethane is a chemical compound which is formally formed by complete ethylation of the hypothetical orthocarbonic acid C(OH)4 (orthocarboxylic acid violates the Erlenmeyer Rule, Erlenmeyer rule and is unstable in free state). History Tetraethoxymethane was described the first time in 1864.H. Bassett, ''Ueber das vierfach-basische kohlensaure Aethyl'', Ann. 132, 54 (1864), . Synthesis The preparation of tetraethoxymethane from the highly toxic trichloronitromethane is known in the literatureEuropäische Patentschrift EP 0881212 B1''Production method of aminobenzene compound'' Erfinder: H. Hashimoto et al., Anmelder: Takeda Chemical Industries, Ltd., veröffentlicht am 30. Oktober 2001. and achieves only yields of 46-49 to 58%: The obvious synthetic route from tetrachloromethane does not provide the desired product, as in the homologous tetramethoxymethane.R.H. De Wolfe, ''Carboxylic ortho acid derivatives: preparation and synthetic applications'', Organic Chemistry, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramethoxymethane
Tetramethoxymethane is a chemical compound which is formally formed by complete methylation of the hypothetical orthocarbonic acid C(OH)4 (orthocarboxylic acid violates the Erlenmeyer Rule, Erlenmeyer rule and is unstable in free state). Preparation The obvious synthetic route from tetrachloromethane does not yield the desired product.R. H. De Wolfe, ''Carboxylic ortho acid derivatives: preparation and synthetic applications'', Organic Chemistry, Vol. 14, Academic Press, Inc. New York – London, 1970, . The original preparation of the tetramethoxymethane was therefore based on chloropicrin: Because of the unpleasant properties of the chloropicrin, other tetrasubstituted reactive methane derivatives were investigated as starting material for tetramethoxymethane. For example, trichloromethanesulfenyl chloride (also used as a chemical warfare agent and easily accessible from Carbon disulfide, carbon bisulfide and chlorine) was used: A less problematic synthesis is based on tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthocarbonic Acid
Orthocarbonic acid (methanetetrol) is the name given to a hypothetical compound with the chemical formula or . Its molecular structure consists of a single carbon atom bonded to four hydroxy groups. It would be therefore a fourfold alcohol. In theory it could lose four protons to give the hypothetical oxocarbon anion (orthocarbonate), and is therefore considered an oxoacid of carbon. The compound has also been given the nickname of "Hitler's Acid" due to the Ball-and-stick model of the compound resembling the Swastika symbol. Orthocarbonic acid is highly unstable. Calculations show that it decomposes spontaneously into carbonic acid and water: : H4CO4 -> H2CO3 + H2O Orthocarbonic acid is one of the group of ''ortho acids'' that have the general structure of .The term ''ortho acid'' is also used to refer to the most hydroxylated acid in a set of oxoacids. Researchers predict that orthocarbonic acid is stable at high pressure; hence it may form in the interior of the ice gia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramethoxymethane
Tetramethoxymethane is a chemical compound which is formally formed by complete methylation of the hypothetical orthocarbonic acid C(OH)4 (orthocarboxylic acid violates the Erlenmeyer Rule, Erlenmeyer rule and is unstable in free state). Preparation The obvious synthetic route from tetrachloromethane does not yield the desired product.R. H. De Wolfe, ''Carboxylic ortho acid derivatives: preparation and synthetic applications'', Organic Chemistry, Vol. 14, Academic Press, Inc. New York – London, 1970, . The original preparation of the tetramethoxymethane was therefore based on chloropicrin: Because of the unpleasant properties of the chloropicrin, other tetrasubstituted reactive methane derivatives were investigated as starting material for tetramethoxymethane. For example, trichloromethanesulfenyl chloride (also used as a chemical warfare agent and easily accessible from Carbon disulfide, carbon bisulfide and chlorine) was used: A less problematic synthesis is based on tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mol (unit)
The mole, symbol mol, is the unit of amount of substance in the International System of Units (SI). The quantity amount of substance is a measure of how many elementary entities of a given substance are in an object or sample. The mole is defined as containing exactly elementary entities. Depending on what the substance is, an elementary entity may be an atom, a molecule, an ion, an ion pair, or a subatomic particle such as an electron. For example, 10 moles of water (a chemical compound) and 10 moles of mercury (a chemical element), contain equal amounts of substance and the mercury contains exactly one atom for each molecule of the water, despite the two having different volumes and different masses. The number of elementary entities in one mole is known as the Avogadro number, which is the approximate number of nucleons (protons or neutrons) in one gram of ordinary matter. The previous definition of a mole was simply the number of elementary entities equal to that of 12 grams ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CH Acidity
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three Covalent bond, bonds) and bears a formal negative charge (in at least one significant resonance form). Formally, a carbanion is the conjugate base of a carbon acid: :R3CH\, + \ddot^- -> \mathbf + HB where B stands for the base. The carbanions formed from deprotonation of alkanes (at an sp3 carbon), alkenes (at an sp2 carbon), arenes (at an sp2 carbon), and alkynes (at an sp carbon) are known as alkyl, alkenyl (Vinyl group, vinyl), aryl, and alkynyl (acetylide) anions, respectively. Carbanions have a concentration of electron density at the negatively charged carbon, which, in most cases, reacts efficiently with a variety of electrophiles of varying strengths, including carbonyl groups, Imine, imines/Iminium, iminium salts, halogenating reagents (e.g., N-Bromosuccinimide, ''N''-bromosuccinimide and Iodine, diiodine), and Brønsted–Lowry acid–base theory, proton donors. A carbanion is one ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Expanding Monomers
Expanding monomers are monomers which increase in volume (expand) during polymerization. They can be added to monomer formulations to counteract the usual volume shrinking (during polymerization) to manufacture products with higher quality and durability. Volume Shrinkage is in first line for the unmeltable thermosets a problem, since those are of fixed shape after polymerization completed. Background The quality of thermosets (crosslinked polymers) is determined by a numerous factors such as the purity of the used monomer, polymerization time and temperature, stoichiometry of comonomers (when used) or type and quantity of catalyst or initiator. Another rarely minded factor is the volume shrinking (and density increase) during polymerization; actually all polymers are shrinking during polymerization to some degree. This volume shrinkage can lead (after the gel point) to mechanical stress within the polymer (internal stress), which may cause microfractures, worse mechanical properti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile. Nomenclature A compound containing the epoxide functional group can be called an epoxy, epoxide, oxirane, and ethoxyline. Simple epoxides are often referred to as oxides. Thus, the epoxide of ethylene (C2H4) is ethylene oxide (C2H4O). Many compounds have trivial names; for instance, ethylene oxide is called "oxirane". Some names emphasize the presence of the epoxide functional group, as in the compound ''1,2-epoxyheptane'', which can also be called ''1,2-heptene oxide''. A polymer formed from epoxide precursors is called an ''epoxy'', but such materials do not contain epoxide groups (or contain only a few residual epoxy grou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spiro Orthocarbonates
Spiro(s) may refer to: * Spiro, Oklahoma, a town in the U.S. ** Spiro Mounds, an archaeological site * Spiro (band), a British music group * Spiro (name), including a list of people with the name * Špiro, South Slavic masculine given name * ARA ''Spiro'', two ships of the Argentine Navy * , an oil tanker * Euler spiral, or spiro, a curve * Spiro compound, a type of chemical structure * Spironolactone, a medicine, often used in feminizing hormone therapy See also * * * Spiro compound, a class of organic compound featuring two rings joined at one atom * Spirou (comics), a Belgian comic strip character * Spyro * Spira (other) {{Disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spiro Compound
In organic chemistry, spiro compounds are compounds that have at least two molecular rings with only one common atom. The simplest spiro compounds are bicyclic (having just two rings), or have a bicyclic portion as part of the larger ring system, in either case with the two rings connected through the defining single common atom. The one common atom connecting the participating rings distinguishes spiro compounds from other bicyclics: from ''isolated ring compounds'' like biphenyl that have no connecting atoms, from ''fused ring compounds'' like decalin having two rings linked by two adjacent atoms, and from ''bridged ring compounds'' like norbornane with two rings linked by two non-adjacent atoms.For all four categories, see The specific chapters can be found aan respectively, same access date. For the description featuring adjacent atoms for all but the isolated category, see Clayden, op. cit. Spiro compounds may be fully carbocyclic (all carbon) or heterocyclic (havi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
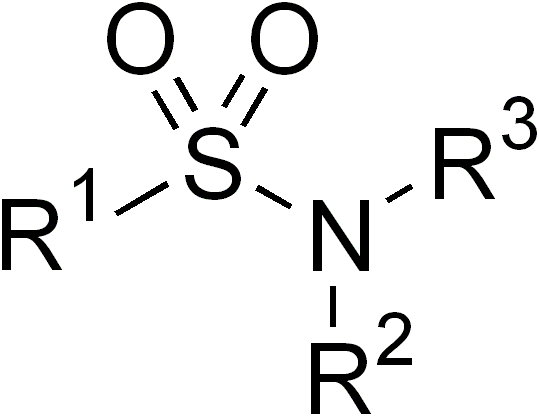
Sulfonamide
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivative or var ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enol Ether
In organic chemistry an enol ether is an alkene with an alkoxy substituent. The general structure is R2C=CR-OR where R = H, alkyl or aryl. A common subfamily of enol ethers are vinyl ethers, with the formula ROCH=CH2. Important enol ethers include the reagent 3,4-dihydropyran and the monomers methyl vinyl ether and ethyl vinyl ether. Reactions and uses Akin to enamines, enol ethers are electron-rich alkenes by virtue of the electron-donation from the heteroatom via pi-bonding. Enol ethers have oxonium ion character. By virtue of their bonding situation, enol ethers display distinctive reactivity. In comparison with simple alkenes, enol ethers exhibit enhanced susceptibility to attack by electrophiles such as Bronsted acids. Similarly, they undergo inverse demand Diels-Alder reactions. The reactivity of enol ethers is highly dependent on the presence of substituents alpha to oxygen. The vinyl ethers are susceptible to polymerization to give polyvinyl ethers. Some vinyl ethers al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |