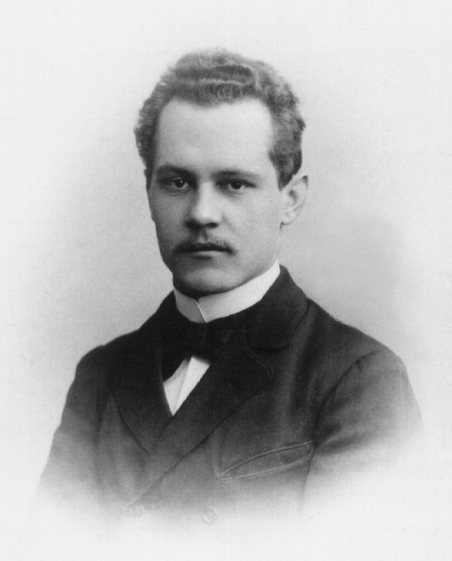|
Tests Of Relativistic Energy And Momentum
Tests of relativistic energy and momentum are aimed at measuring the relativistic expressions for energy, momentum, and mass. According to special relativity, the properties of particles moving approximately at the speed of light significantly deviate from the predictions of Newtonian mechanics. For instance, the speed of light cannot be reached by massive particles. Today, those relativistic expressions for particles close to the speed of light are routinely confirmed in undergraduate laboratories, and necessary in the design and theoretical evaluation of collision experiments in particle accelerators. See also Tests of special relativity for a general overview. Overview In classical mechanics, kinetic energy and momentum are expressed as :E_=\tfracmv^ ,\quad p=mv . \, On the other hand, special relativity predicts that the speed of light is constant in all inertial frames of references. The relativistic energy–momentum relation reads: :E^-(pc)^=(mc^)^ \,, from which the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Relativistic Mass
The word "mass" has two meanings in special relativity: ''invariant mass'' (also called rest mass) is an invariant quantity which is the same for all observers in all reference frames, while the relativistic mass is dependent on the velocity of the observer. According to the concept of mass–energy equivalence, invariant mass is equivalent to '' rest energy'', while relativistic mass is equivalent to '' relativistic energy'' (also called total energy). The term "relativistic mass" tends not to be used in particle and nuclear physics and is often avoided by writers on special relativity, in favor of referring to the body's relativistic energy. In contrast, "invariant mass" is usually preferred over rest energy. The measurable inertia of a body in a given frame of reference is determined by its relativistic mass, not merely its invariant mass. For example, photons have zero rest mass but contribute to the inertia (and weight in a gravitational field) of any system containing th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Karl Glitscher
Karl Glitscher (1886 – 1945) was a German physicist who made contributions to quantum mechanics. Education Glitscher studied under Arnold Sommerfeld at the Ludwig Maximilian University of Munich. For his doctoral dissertation, Sommerfeld asked Glitscher to compare the relativistic theory of the electron with Max Abraham's theory of the rigid electron relative to the fine structure of spectral lines. Following a suggestion by Wilhelm Lenz, a former student of Sommerfeld who received his doctorate in 1911, Glitscher was able to calculate the fine structure spectra and he found that the rigid electron was ruled out by Friedrich Paschen's data on one-electron atoms and the X-ray spectral doublets. Glitscher's theoretical calculations served as his doctoral thesis, and he was awarded his doctorate in 1917. Career Glitscher filed a patent in Germany in 1930 and in the United States in 1931 for an artificial horizon indicator for vehicles or similar platforms. The patent was as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Old Quantum Theory
The old quantum theory is a collection of results from the years 1900–1925, which predate modern quantum mechanics. The theory was never complete or self-consistent, but was instead a set of heuristic corrections to classical mechanics. The theory has come to be understood as the semi-classical approximation to modern quantum mechanics. The main and final accomplishments of the old quantum theory were the determination of the modern form of the periodic table by Edmund Stoner and the Pauli exclusion principle, both of which were premised on Arnold Sommerfeld's enhancements to the Bohr model of the atom. The main tool of the old quantum theory was the Bohr–Sommerfeld quantization condition, a procedure for selection of certain allowed states of a classical system: the system can then only exist in one of the allowed states and not in any other state. History The old quantum theory was instigated by the 1900 work of Max Planck on the emission and absorption of light in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Spectral Series
The emission spectrum of atomic hydrogen has been divided into a number of ''spectral series'', with wavelengths given by the Rydberg formula. These observed spectral lines are due to the electron making transitions between two energy levels in an atom. The classification of the series by the Rydberg formula was important in the development of quantum mechanics. The spectral series are important in astronomical spectroscopy for detecting the presence of hydrogen and calculating red shifts. Physics A hydrogen atom consists of an electron orbiting its nucleus. The electromagnetic force between the electron and the nuclear proton leads to a set of quantum states for the electron, each with its own energy. These states were visualized by the Bohr model of the hydrogen atom as being distinct orbits around the nucleus. Each energy level, or electron shell, or orbit, is designated by an integer, as shown in the figure. The Bohr model was later replaced by quantum mechanics in whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fine Structure
In atomic physics, the fine structure describes the splitting of the spectral lines of atoms due to electron spin and relativistic corrections to the non-relativistic Schrödinger equation. It was first measured precisely for the hydrogen atom by Albert A. Michelson and Edward W. Morley in 1887, laying the basis for the theoretical treatment by Arnold Sommerfeld, introducing the fine-structure constant. Background Gross structure The ''gross structure'' of line spectra is the structure predicted by the quantum mechanics of non-relativistic electrons with no spin. For a hydrogenic atom, the gross structure energy levels only depend on the principal quantum number ''n''. However, a more accurate model takes into account relativistic and spin effects, which break the degeneracy of the energy levels and split the spectral lines. The scale of the fine structure splitting relative to the gross structure energies is on the order of (''Zα'')2, where ''Z'' is the atomic number ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arnold Sommerfeld
Arnold Johannes Wilhelm Sommerfeld (; 5 December 1868 – 26 April 1951) was a German Theoretical physics, theoretical physicist who pioneered developments in Atomic physics, atomic and Quantum mechanics, quantum physics, and also educated and mentored many students for the new era of theoretical physics. He served as doctoral advisor and Postdoctoral researcher, postdoc advisor to seven Nobel Prize winners and supervised at least 30 other famous physicists and chemists. Only J. J. Thomson's record of mentorship offers a comparable list of high-achieving students. He introduced the second quantum number, azimuthal quantum number, and the third quantum number, magnetic quantum number. He also introduced the fine-structure constant and pioneered X-ray wave theory. Early life and education Sommerfeld was born in 1868 to a family with deep ancestral roots in Prussia. His mother Cäcilie Matthias (1839–1902) was the daughter of a Potsdam builder. His father Franz Sommerfeld (1820� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Max Abraham
Max Abraham (; 26 March 1875 – 16 November 1922) was a German physicist known for his work on electromagnetism and his opposition to the theory of relativity. Biography Abraham was born in Danzig, Imperial Germany (now Gdańsk in Poland) to a family of Jewish merchants. His father was Moritz Abraham and his mother was Selma Moritzsohn. Attending the University of Berlin, he studied under Max Planck. He graduated in 1897. For the next three years, Abraham worked as Planck's assistant. From 1900 to 1909, Abraham worked at Göttingen as a privatdozent, an unpaid lecturing position. Abraham developed his theory of the electron in 1902, in which he hypothesized that the electron was a perfect sphere with a charge divided evenly around its surface. Abraham's model was competing with that developed by Hendrik Lorentz (1899, 1904) and Albert Einstein (1905) which seem to have become more widely accepted; nevertheless, Abraham never gave up his model, since he considered it wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electromagnetic Mass
Electromagnetic mass was initially a concept of classical mechanics, denoting as to how much the electromagnetic field, or the self-energy, is contributing to the mass of charged particles. It was first derived by J. J. Thomson in 1881 and was for some time also considered as a dynamical explanation of inertial mass ''per se''. Today, the relation of mass, momentum, velocity, and all forms of energy – including electromagnetic energy – is analyzed on the basis of Albert Einstein's special relativity and mass–energy equivalence. As to the cause of mass of elementary particles, the Higgs mechanism in the framework of the relativistic Standard Model is currently used. However, some problems concerning the electromagnetic mass and self-energy of charged particles are still studied. Charged particles Rest mass and energy It was recognized by J. J. Thomson in 1881 that a charged sphere moving in a space filled with a medium of a specific inductive capacity (the electromagne ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass-to-charge Ratio
The mass-to-charge ratio (''m''/''Q'') is a physical quantity Ratio, relating the ''mass'' (quantity of matter) and the ''electric charge'' of a given particle, expressed in Physical unit, units of kilograms per coulomb (kg/C). It is most widely used in the electrodynamics of charged particles, e.g. in electron optics and ion optics. It appears in the scientific fields of electron microscopy, cathode ray tubes, accelerator physics, nuclear physics, Auger electron spectroscopy, physical cosmology, cosmology and mass spectrometry. The importance of the mass-to-charge ratio, according to classical electrodynamics, is that two particles with the same mass-to-charge ratio move in the same path in a vacuum, when subjected to the same electric and magnetic fields. Some disciplines use the charge-to-mass ratio (''Q''/''m'') instead, which is the multiplicative inverse of the mass-to-charge ratio. The CODATA recommended value for an electron is Origin When charged particles move in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Ray
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus, known as beta decay. There are two forms of beta decay, β− decay and β+ decay, which produce electrons and positrons, respectively. Beta particles with an energy of 0.5 MeV have a range of about one metre in the air; the distance is dependent on the particle's energy and the air's density and composition. Beta particles are a type of ionizing radiation, and for radiation protection purposes, they are regarded as being more ionising than gamma rays, but less ionising than alpha particles. The higher the ionising effect, the greater the damage to living tissue, but also the lower the penetrating power of the radiation through matter. Beta decay modes β− decay (electron emission) An unstable atomic nucleus with an excess of neutrons may undergo β− decay, where a neutron is converted into a proto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |