|
System (thermodynamics)
A thermodynamic system is a body of matter and/or radiation, confined in space by walls, with defined permeabilities, which separate it from its surroundings. The surroundings may include other thermodynamic systems, or physical systems that are not thermodynamic systems. A wall of a thermodynamic system may be purely notional, when it is described as being 'permeable' to all matter, all radiation, and all forces. A state of a thermodynamic system can be fully described in several different ways, by several different sets of thermodynamic state variables. A widely used distinction is between ''isolated'', ''closed'', and ''open'' thermodynamic systems. An isolated thermodynamic system has walls that are non-conductive of heat and perfectly reflective of all radiation, that are rigid and immovable, and that are impermeable to all forms of matter and all forces. (Some writers use the word 'closed' when here the word 'isolated' is being used.) A closed thermodynamic system is c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diagram Systems
A diagram is a symbolic Depiction, representation of information using Visualization (graphics), visualization techniques. Diagrams have been used since prehistoric times on Cave painting, walls of caves, but became more prevalent during the Age of Enlightenment, Enlightenment. Sometimes, the technique uses a Three-dimensional space, three-dimensional visualization which is then graphical projection, projected onto a two-dimensional surface. The word ''graphics, graph'' is sometimes used as a synonym for diagram. Overview The term "diagram" in its commonly used sense can have a general or specific meaning: * ''visual information device'' : Like the term "illustration", "diagram" is used as a collective term standing for the whole class of technical genres, including graphics, graphs, technical drawings and tables. * ''specific kind of visual display'' : This is the genre that shows qualitative data with shapes that are connected by lines, arrows, or other visual links. In sci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the microscopic description of nature in statistical physics, and to the principles of information theory. It has found far-ranging applications in chemistry and physics, in biological systems and their relation to life, in cosmology, economics, sociology, weather science, climate change, and information systems including the transmission of information in telecommunication. The thermodynamic concept was referred to by Scottish scientist and engineer William Rankine in 1850 with the names ''thermodynamic function'' and ''heat-potential''. In 1865, German physicist Rudolf Clausius, one of the leading founders of the field of thermodynamics, defined it as the quotient of an infinitesimal amount of hea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mechanical Work
In physics, work is the energy transferred to or from an object via the application of force along a displacement. In its simplest form, for a constant force aligned with the direction of motion, the work equals the product of the force strength and the distance traveled. A force is said to do ''positive work'' if when applied it has a component in the direction of the displacement of the point of application. A force does ''negative work'' if it has a component opposite to the direction of the displacement at the point of application of the force. For example, when a ball is held above the ground and then dropped, the work done by the gravitational force on the ball as it falls is positive, and is equal to the weight of the ball (a force) multiplied by the distance to the ground (a displacement). If the ball is thrown upwards, the work done by its weight is negative, and is equal to the weight multiplied by the displacement in the upwards direction. When the force is consta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
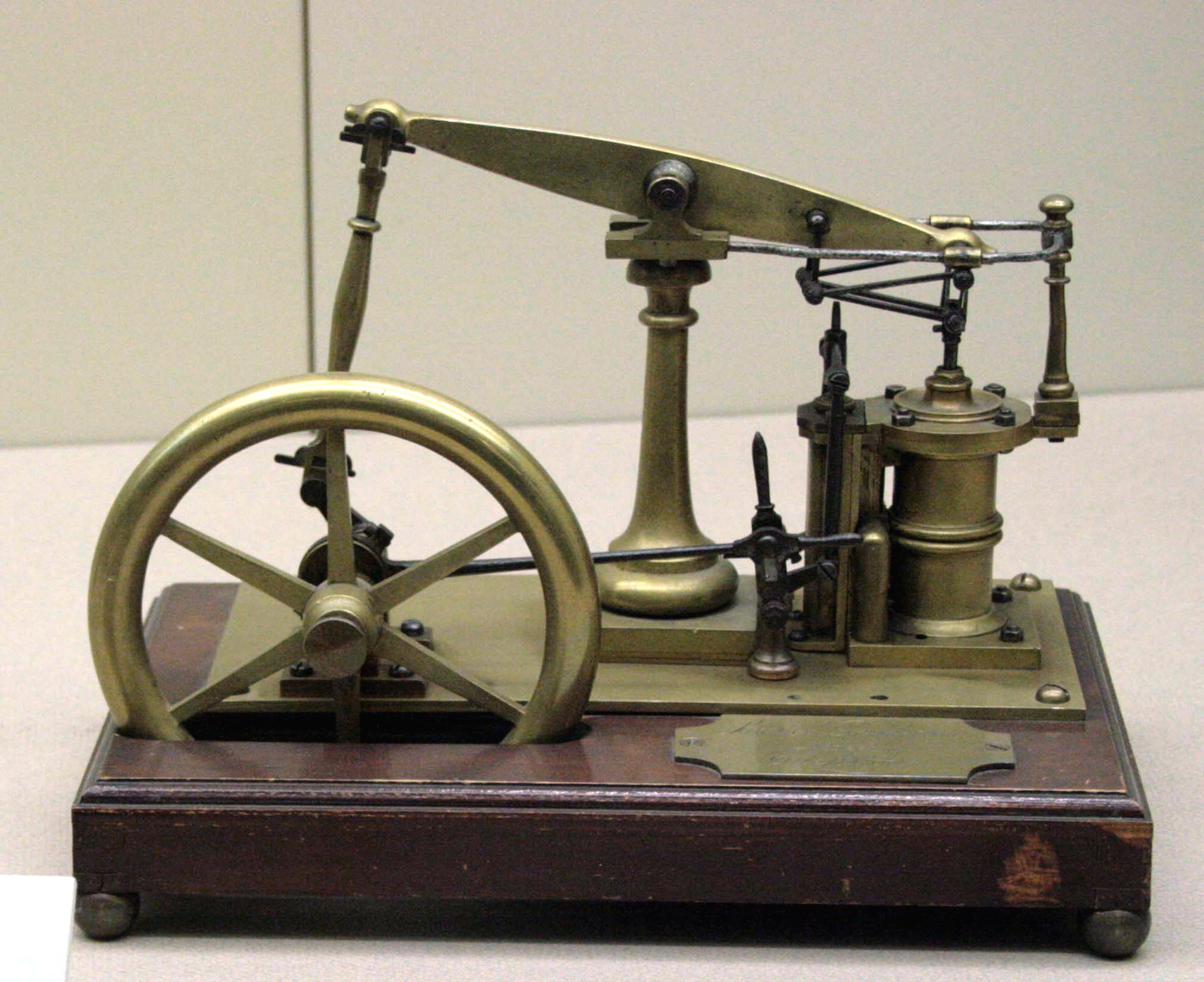
Carnot Heat Engine 2
Carnot may refer to: People *Carnot Posey (1818–1863), American lawyer and military officer People with the surname *Lazare Carnot (1753-1823), French mathematician and politician of the French Revolution *Louis Carnot (born 2001), French French footballer *Nicolas Léonard Sadi Carnot (1796-1832), French military scientist and physicist; son of Lazare Carnot *Hippolyte Carnot (1801-1888), French politician; son of Lazare Carnot *Marie François Sadi Carnot (1837-1894), French politician; President of France from 1887 to 1894 and son of Hippolyte Carnot * Marie-Adolphe Carnot (1839-1920), French mining engineer and chemist; son of Hippolyte Carnot *Paul Carnot (1869-1957), French physician; son of Marie-Adolphe Carnot *Stéphane Carnot (born 1972), former French footballer Places *Carnot, Central African Republic, a city *Carnot, Wisconsin, United States *Carnot-Moon, Pennsylvania, United States Other uses *Carnot cycle, in thermodynamics *Carnot heat engine, an idealised the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carnot Heat Engine
A Carnot heat engine is a heat engine that operates on the Carnot cycle. The basic model for this engine was developed by Nicolas Léonard Sadi Carnot in 1824. The Carnot engine model was graphically expanded by Benoît Paul Émile Clapeyron in 1834 and mathematically explored by Rudolf Clausius in 1857, work that led to the fundamental thermodynamic concept of entropy. The Carnot engine is the most efficient heat engine which is theoretically possible. The efficiency depends only upon the absolute temperatures of the hot and cold heat reservoirs between which it operates. A heat engine acts by transferring energy from a warm region to a cool region of space and, in the process, converting some of that energy to mechanical work. The cycle may also be reversed. The system may be worked upon by an external force, and in the process, it can transfer thermal energy from a cooler system to a warmer one, thereby acting as a refrigerator or heat pump rather than a heat engine. Every th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is also often used to refer to the thermal energy contained in a system as a component of its internal energy and that is reflected in the temperature of the system. For both uses of the term, heat is a form of energy. An example of formal vs. informal usage may be obtained from the right-hand photo, in which the metal bar is "conducting heat" from its hot end to its cold end, but if the metal bar is considered a thermodynamic system, then the energy flowing within the metal bar is called internal energy, not heat. The hot metal bar is also transferring heat to its surroundings, a correct statement for both the strict and loose meanings of ''heat''. Another example of informal usage is the term '' heat content'', used despite the fact that p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Work (thermodynamics)
In thermodynamics, work is one of the principal processes by which a thermodynamic system can interact with its surroundings and exchange energy. An exchange of energy is facilitated by a mechanism through which the system can spontaneously exert macroscopic forces on its surroundings, or vice versa. In the surroundings, this mechanical work can lift a weight, for example. The externally measured forces and external effects may be electromagnetic,Guggenheim, E.A. (1985). ''Thermodynamics. An Advanced Treatment for Chemists and Physicists'', seventh edition, North Holland, Amsterdam, .Jackson, J.D. (1975). ''Classical Electrodynamics'', second edition, John Wiley and Sons, New York, .Konopinski, E.J. (1981). ''Electromagnetic Fields and Relativistic Particles'', McGraw-Hill, New York, . gravitational,North, G.R., Erukhimova, T.L. (2009). ''Atmospheric Thermodynamics. Elementary Physics and Chemistry'', Cambridge University Press, Cambridge (UK), . or mechanical (such as pressure-v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rudolf Clausius
Rudolf Julius Emanuel Clausius (; 2 January 1822 – 24 August 1888) was a German physicist and mathematician and is considered one of the central founding fathers of the science of thermodynamics. By his restatement of Sadi Carnot's principle known as the Carnot cycle, he gave the theory of heat a truer and sounder basis. His most important paper, "On the Moving Force of Heat", published in 1850, first stated the basic ideas of the second law of thermodynamics. In 1865 he introduced the concept of entropy. In 1870 he introduced the virial theorem, which applied to heat. Life Clausius was born in Köslin (now Koszalin, Poland) in the Province of Pomerania in Prussia. His father was a Protestant pastor and school inspector, and Rudolf studied in the school of his father. In 1838, he went to the Gymnasium in Stettin. Clausius graduated from the University of Berlin in 1844 where he had studied mathematics and physics since 1840 with, among others, Gustav Magnus, Peter Gustav Le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steam Engine
A steam engine is a heat engine that performs mechanical work using steam as its working fluid. The steam engine uses the force produced by steam pressure to push a piston back and forth inside a cylinder. This pushing force can be transformed, by a connecting rod and crank, into rotational force for work. The term "steam engine" is generally applied only to reciprocating engines as just described, not to the steam turbine. Steam engines are external combustion engines, where the working fluid is separated from the combustion products. The ideal thermodynamic cycle used to analyze this process is called the Rankine cycle. In general usage, the term ''steam engine'' can refer to either complete steam plants (including boilers etc.), such as railway steam locomotives and portable engines, or may refer to the piston or turbine machinery alone, as in the beam engine and stationary steam engine. Although steam-driven devices were known as early as the aeolipile in the f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reflections On The Motive Power Of Fire
''Reflections on the Motive Power of Fire and on Machines Fitted to Develop that Power'' is a book published in 1824 by French physicist Sadi Carnot.full text of 1897 ed. ( Full text of 1897 edition on Wikisource ) The 118-page book's French title was ''Réflexions sur la puissance motrice du feu et sur les machines propres à développer cette puissance''. It is a significant publication in the history of thermodynamics about a generalized theory of heat engines. Overview The book is considered the founding work of thermodynamics. It contains the preliminary outline of the second law of thermodynamics. Carnot stated that motive power is due to the fall of caloric (heat) from a hot to a cold body. The work was unnoticed until 1834 when French mining engineer Émile Clapeyron put it on a graphical footing in his ''Memoir on the Motive Power of Heat''. Through Clapeyron's paper, German physicist Rudolf Clausius learned of Carnot's theory of heat and through a modification of Ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
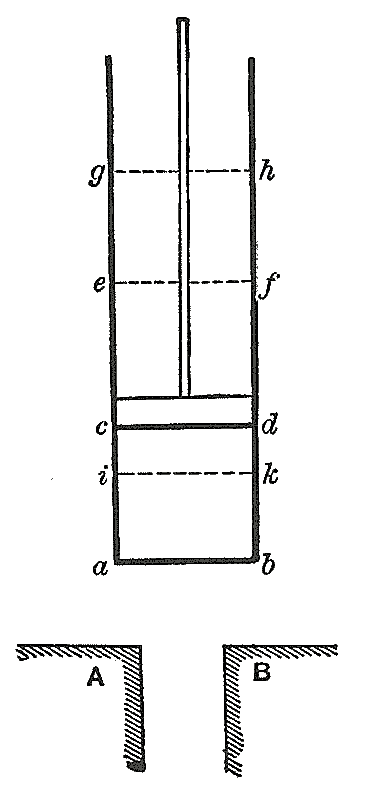
Flow Process
The region of space enclosed by open system boundaries is usually called a control volume. It may or may not correspond to physical walls. It is convenient to define the shape of the control volume so that all flow of matter, in or out, occurs perpendicular to its surface. One may consider a process in which the matter flowing into and out of the system is chemically homogeneous.Shavit, A., Gutfinger, C. (1995). ''Thermodynamics. From Concepts to Applications'', Prentice Hall, London, , Chapter 6. Then the inflowing matter performs work as if it were driving a piston of fluid into the system. Also, the system performs work as if it were driving out a piston of fluid. Through the system walls that do not pass matter, heat () and work () transfers may be defined, including shaft work. Classical thermodynamics considers processes for a system that is initially and finally in its own internal state of thermodynamic equilibrium, with no flow. This is feasible also under some restrict ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
State Function
In the thermodynamics of equilibrium, a state function, function of state, or point function for a thermodynamic system is a mathematical function relating several state variables or state quantities (that describe equilibrium states of a system) that depend only on the current equilibrium thermodynamic state of the system (e.g. gas, liquid, solid, crystal, or emulsion), not the path which the system has taken to reach that state. A state function describes equilibrium states of a system, thus also describing the type of system. A state variable is typically a state function so the determination of other state variable values at an equilibrium state also determines the value of the state variable as the state function at that state. The ideal gas law is a good example. In this law, one state variable (e.g., pressure, volume, temperature, or the amount of substance in a gaseous equilibrium system) is a function of other state variables so is regarded as a state function. A state fu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




.png)