|
Superchlorination
Shock chlorination is a process used in many swimming pools, water wells, springs, and other water sources to reduce the bacterial and algal residue in the water. Shock chlorination is performed by mixing a large amount of sodium hypochlorite, which can be in the form of a powder or a liquid such as chlorine bleach, into the water. The common advice is that the amount added must raise the level of chlorine to 10X the level (in parts per million) of chloramines present in the pool water; this is "shocking". A lesser ratio is termed superchlorinating. Water that is being shock chlorinated should not be swum in or drunk until the sodium hypochlorite count in the water goes down to three ppm or less. Commercial sodium hypochlorite should not be mixed with commercial calcium hypochlorite, as there is a risk of explosion. Although a verb for superchlorination, "shock" is often misunderstood (through marketing and sales language) to be a unique type of product. Drawbacks While "shock ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Well
A well is an excavation or structure created in the ground by digging, driving, or drilling to access liquid resources, usually water. The oldest and most common kind of well is a water well, to access groundwater in underground aquifers. The well water is drawn up by a pump, or using containers, such as buckets or large water bags that are raised mechanically or by hand. Water can also be injected back into the aquifer through the well. Wells were first constructed at least eight thousand years ago and historically vary in construction from a simple scoop in the sediment of a dry watercourse to the qanats of Iran, and the stepwells and sakiehs of India. Placing a lining in the well shaft helps create stability, and linings of wood or wickerwork date back at least as far as the Iron Age. Wells have traditionally been sunk by hand digging, as is still the case in rural areas of the developing world. These wells are inexpensive and low-tech as they use mostly manual labo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spring (hydrosphere)
A spring is a point of exit at which groundwater from an aquifer flows out on top of Earth's crust ( pedosphere) and becomes surface water. It is a component of the hydrosphere. Springs have long been important for humans as a source of fresh water, especially in arid regions which have relatively little annual rainfall. Springs are driven out onto the surface by various natural forces, such as gravity and hydrostatic pressure. Their yield varies widely from a volumetric flow rate of nearly zero to more than for the biggest springs. Formation Springs are formed when groundwater flows onto the surface. This typically happens when the groundwater table reaches above the surface level. Springs may also be formed as a result of karst topography, aquifers, or volcanic activity. Springs also have been observed on the ocean floor, spewing hot water directly into the ocean. Springs formed as a result of karst topography create karst springs, in which ground water travels throu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
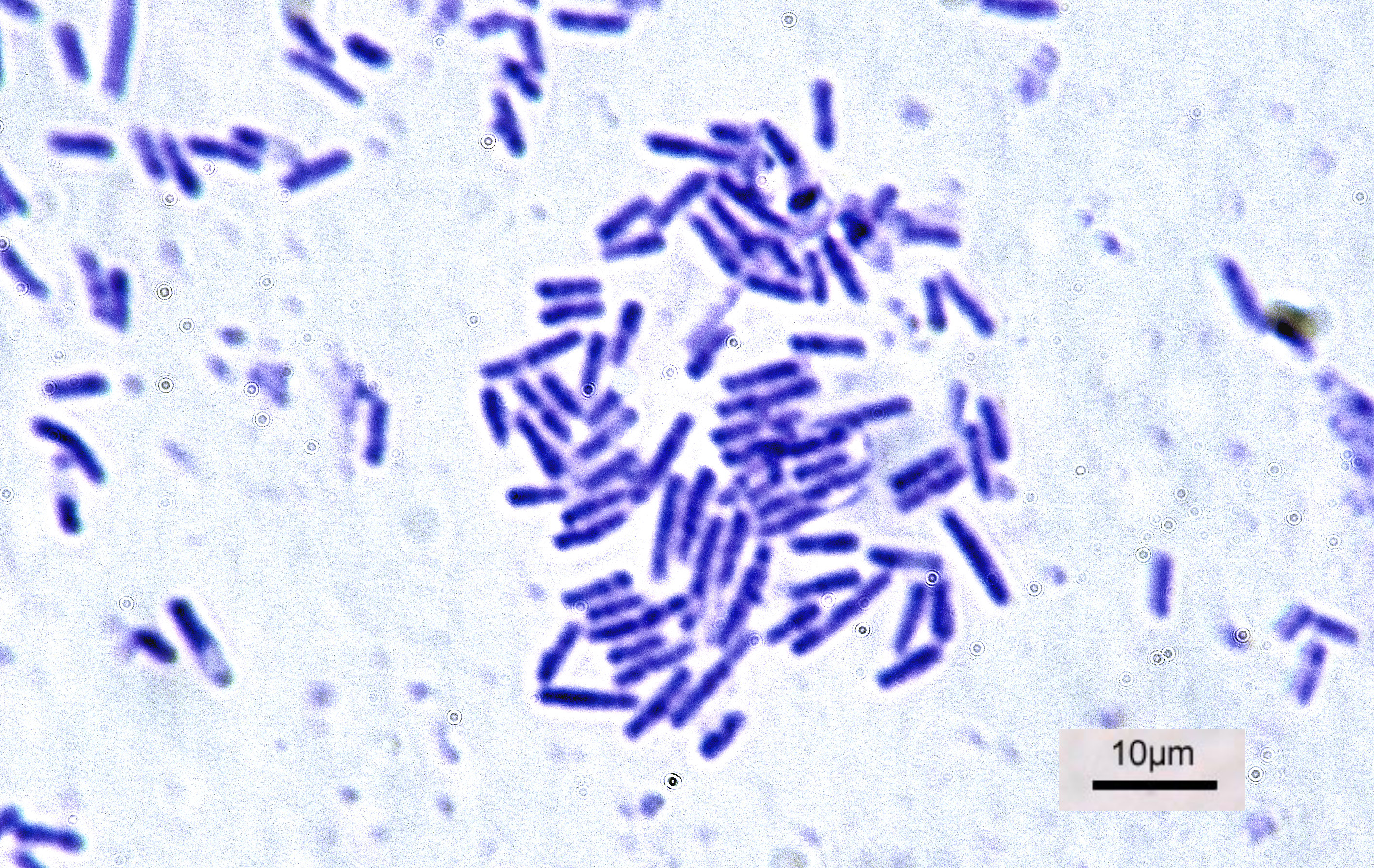
Bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among the first life forms to appear on Earth, and are present in most of its habitats. Bacteria inhabit soil, water, Hot spring, acidic hot springs, radioactive waste, and the deep biosphere of Earth's crust. Bacteria are vital in many stages of the nutrient cycle by recycling nutrients such as the nitrogen fixation, fixation of nitrogen from the Earth's atmosphere, atmosphere. The nutrient cycle includes the decomposition of cadaver, dead bodies; bacteria are responsible for the putrefaction stage in this process. In the biological communities surrounding hydrothermal vents and cold seeps, extremophile bacteria provide the nutrients needed to sustain life by converting dissolved compounds, such as hydrogen sulp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Algae
Algae (; singular alga ) is an informal term for a large and diverse group of photosynthetic eukaryotic organisms. It is a polyphyletic grouping that includes species from multiple distinct clades. Included organisms range from unicellular microalgae, such as ''Chlorella,'' ''Prototheca'' and the diatoms, to multicellular forms, such as the giant kelp, a large brown alga which may grow up to in length. Most are aquatic and autotrophic (they generate food internally) and lack many of the distinct cell and tissue types, such as stomata, xylem and phloem that are found in land plants. The largest and most complex marine algae are called seaweeds, while the most complex freshwater forms are the ''Charophyta'', a division of green algae which includes, for example, ''Spirogyra'' and stoneworts. No definition of algae is generally accepted. One definition is that algae "have chlorophyll ''a'' as their primary photosynthetic pigment and lack a sterile covering of cells around their re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hypochlorite
Sodium hypochlorite (commonly known in a dilute solution as bleach) is an inorganic chemical compound with the formula NaOCl (or NaClO), comprising a sodium cation () and a hypochlorite anion (or ). It may also be viewed as the sodium salt of hypochlorous acid. The anhydrous compound is unstable and may decompose explosively. It can be crystallized as a pentahydrate ·5, a pale greenish-yellow solid which is not explosive and is stable if kept refrigerated. Sodium hypochlorite is most often encountered as a pale greenish-yellow dilute solution referred to as liquid bleach, which is a household chemical widely used (since the 18th century) as a disinfectant or a bleaching agent. In solution, the compound is unstable and easily decomposes, liberating chlorine, which is the active principle of such products. Sodium hypochlorite is the oldest and still most important chlorine-based bleach. Its corrosive properties, common availability, and reaction products make it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine Bleach
Bleach is the generic name for any chemical product that is used industrially or domestically to remove color (whitening) from a fabric or fiber or to clean or to remove stains in a process called bleaching. It often refers specifically, to a dilute solution of sodium hypochlorite, also called "liquid bleach". Many bleaches have broad spectrum bactericidal properties, making them useful for disinfecting and sterilizing. They are used in swimming pool sanitation to control bacteria, viruses, and algae, and in many places where sterile conditions are required. They are also used in many industrial processes, notably in the bleaching of wood pulp. Bleaches also have other minor uses like removing mildew, killing weeds, and increasing the longevity of cut flowers. Bleaches work by reacting with many colored organic compounds, such as natural pigments, and turning them into colorless ones. While most bleaches are oxidizing agents (chemicals that can remove electrons from oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloramines
Chloramines refer to derivatives of ammonia and organic amines wherein one or more N-H bonds have been replaced by N-Cl bonds. Two classes of compounds are considered: inorganic chloramines and organic chloramines. Inorganic chloramines Inorganic chloramines comprise three compounds: monochloramine (NH2Cl), dichloramine (NHCl2), and nitrogen trichloride (NCl3). Monochloramine is of broad significance as a disinfectant for water. Organic chloramines 144px, ''N''-Chloropiperidine is a rare example of an organic chloramine. 144px, Chloramine-T is often referred to as a chloramine, but it is really a salt (CH3C6H4SO2NClNa) derived from a chloramine. Organic chloramines feature the NCl functional group In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the re ... attached to an organic s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parts Per Million
In science and engineering, the parts-per notation is a set of pseudo-units to describe small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction. Since these fractions are quantity-per-quantity measures, they are pure numbers with no associated units of measurement. Commonly used are parts-per-million (ppm, ), parts-per-billion (ppb, ), parts-per-trillion (ppt, ) and parts-per-quadrillion (ppq, ). This notation is not part of the International System of Units (SI) system and its meaning is ambiguous. Overview Parts-per notation is often used describing dilute solutions in chemistry, for instance, the relative abundance of dissolved minerals or pollutants in water. The quantity "1 ppm" can be used for a mass fraction if a water-borne pollutant is present at one-millionth of a gram per gram of sample solution. When working with aqueous solutions, it is common to assume that the density of water is 1.00 g/mL. Therefore, it is common to eq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Hypochlorite
Calcium hypochlorite is an inorganic compound with formula Ca(OCl)2. It is the main active ingredient of commercial products called bleaching powder, chlorine powder, or chlorinated lime, used for water treatment and as a bleaching agent. This compound is relatively stable and has greater available chlorine than sodium hypochlorite. It is a white solid, although commercial samples appear yellow. It strongly smells of chlorine, owing to its slow decomposition in moist air. History Charles Tennant and Charles Macintosh developed an industrial process for the manufacture of Chloride of Lime in the late 18th Century. It was patented in 1799 and used heavily during World War I for disinfecting the trenches and wounds. Uses Sanitation Calcium hypochlorite is commonly used to sanitize public swimming pools and disinfect drinking water. Generally the commercial substances are sold with a purity of 65% to 73% with other chemicals present, such as calcium chloride and calcium carbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Creatinine
Creatinine (; ) is a breakdown product of creatine phosphate from muscle and protein metabolism. It is released at a constant rate by the body (depending on muscle mass). Biological relevance Serum creatinine (a blood measurement) is an important indicator of kidney health, because it is an easily measured byproduct of muscle metabolism that is excreted unchanged by the kidneys. Creatinine itself is produced via a biological system involving creatine, phosphocreatine (also known as creatine phosphate), and adenosine triphosphate (ATP, the body's immediate energy supply). Creatine is synthesized primarily in the liver from the methylation of glycocyamine (guanidino acetate, synthesized in the kidney from the amino acids arginine and glycine) by S-Adenosyl methionine. It is then transported through blood to the other organs, muscle, and brain, where, through phosphorylation, it becomes the high-energy compound phosphocreatine. Creatine conversion to phosphocreatine is catalyzed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Human Sweat
Perspiration, also known as sweating, is the production of fluids secreted by the sweat glands in the skin of mammals. Two types of sweat glands can be found in humans: eccrine glands and apocrine glands. The eccrine sweat glands are distributed over much of the body and are responsible for secreting the watery, brackish sweat most often triggered by excessive body temperature. The apocrine sweat glands are restricted to the armpits and a few other areas of the body and produce an odorless, oily, opaque secretion which then gains its characteristic odor from bacterial decomposition. In humans, sweating is primarily a means of thermoregulation, which is achieved by the water-rich secretion of the eccrine glands. Maximum sweat rates of an adult can be up to 2–4 liters per hour or 10–14 liters per day (10–15 g/min·m2), but is less in children prior to puberty. Evaporation of sweat from the skin surface has a cooling effect due to evaporative cooling. Hence, in hot weath ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disinfection Byproducts
Disinfection by-products (DBPs) result from chemical reactions between organic and inorganic matter in water with chemical treatment agents during the water disinfection process. Chlorination disinfection byproducts Chlorinated disinfection agents such as chlorine and monochloramine are strong oxidizing agents introduced into water in order to destroy pathogenic microbes, to oxidize taste/odor-forming compounds, and to form a disinfectant residual so water can reach the consumer tap safe from microbial contamination. These disinfectants may react with naturally present fulvic and humic acids, amino acids, and other natural organic matter, as well as iodide and bromide ions, to produce a range of DBPs such as the trihalomethanes (THMs), haloacetic acids (HAAs), bromate, and chlorite (which are regulated in the US), and so-called "emerging" DBPs such as halonitromethanes, haloacetonitriles, haloamides, halofuranones, iodo-acids such as iodoacetic acid, iodo-THMs ( iodotriha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




.jpg)

