|
Sulfated Tyrosine
Tyrosine sulfation is a posttranslational modification where a sulfate group is added to a tyrosine residue of a protein molecule. Secreted proteins and extracellular parts of membrane proteins that pass through the Golgi apparatus may be sulfated. Sulfation was first discovered by Bettelheim in bovine fibrinopeptide B in 1954 and later found to be present in animals and plants but not in prokaryotes or in yeast. Function Sulfation plays a role in strengthening protein-protein interactions. Types of human proteins known to undergo tyrosine sulfation include adhesion molecules, G-protein-coupled receptors, coagulation factors, serine protease inhibitors, extracellular matrix proteins, and hormones. Tyrosine O-sulfate is a stable molecule and is excreted in urine in animals. No enzymatic mechanism of tyrosine sulfate desulfation is known to exist. By knock-out of TPST genes in mice, it may be observed that tyrosine sulfation has effects on the growth of the mice, such as body weight, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Posttranslational Modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important components in cell signal transduction, signaling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C-terminus, C- or N-terminus, N- termini. They can extend the chemical repertoire of the 20 standard amino acids by modifying an existing functional group or introducing a new one such as phosphorylation, phosphate. Phosphorylation is a highly effective mechanism for regulating the activity of enzymes and is the most common post-translational modification. Many eukaryotic and prokaryotic proteins also have carbohydra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid. Spelling "Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English. Structure The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry is the same as that of methane. The sulfur atom is in the +6 oxidation state while the four oxygen atoms are each in the −2 state. The sulfate ion carries an overall charge of −2 and it is the conjugate base of the bisulfate (or hydrogensulfate) ion, , which is in turn the conjugate base of , sulfuric acid. Organic sulfate esters, such as dimethyl sulfate, are covalent compounds and esters of sulfuric acid. The tetrahedral molecular geometry of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek ''tyrós'', meaning ''cheese'', as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a Hydrophobe, hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is Genetic code, encoded by the Genetic code#Codons, codons UAC and UAU in messenger RNA. Functions Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of the phenol functionality. It occurs in proteins that are part of signal transduction processes and functions as a receiver of phosphate groups that are transferred by way of protein kinases. Phosphorylation of the hyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
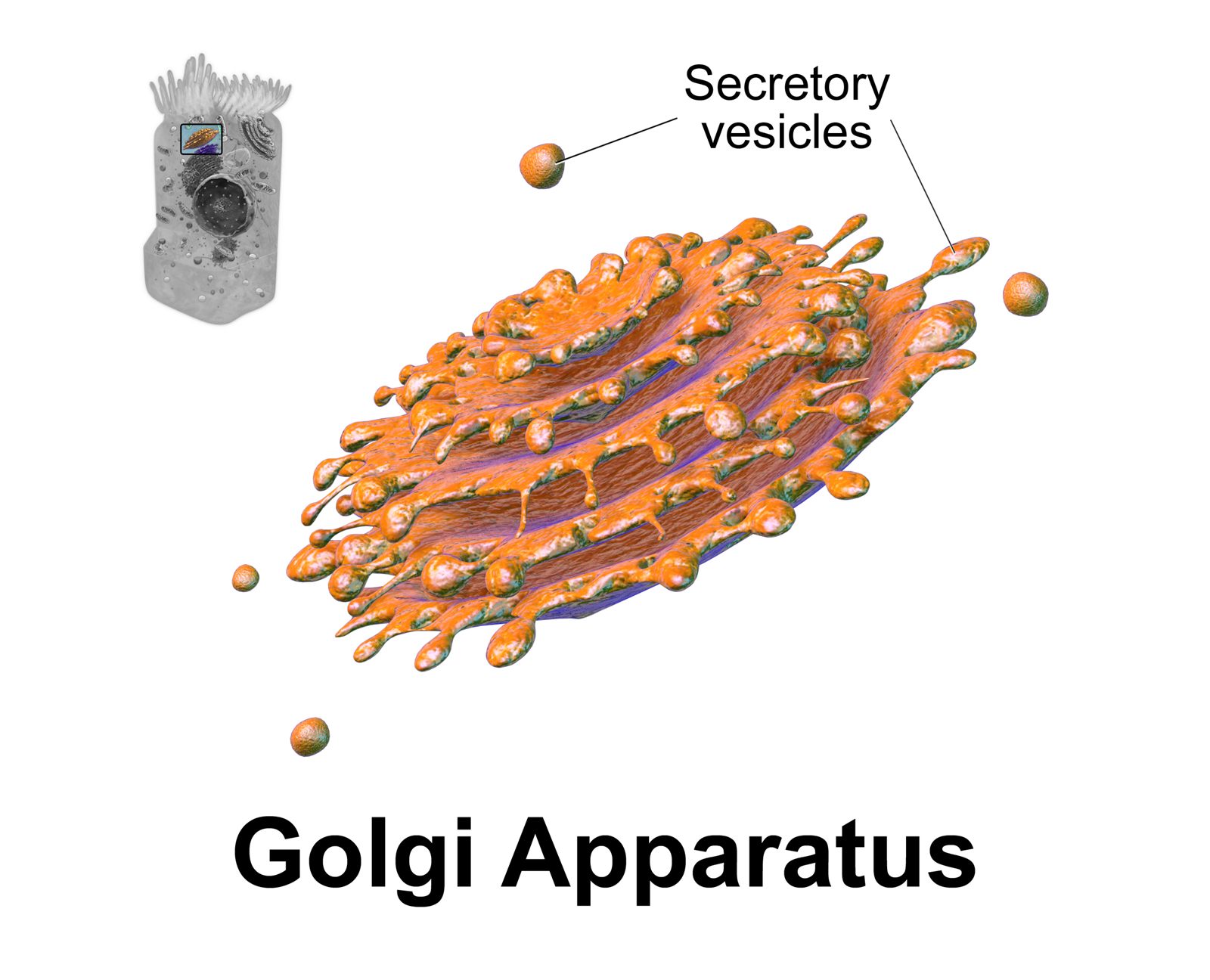
Golgi Apparatus
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus. It was identified in 1897 by the Italian scientist Camillo Golgi and was named after him in 1898. Discovery Owing to its large size and distinctive structure, the Golgi apparatus was one of the first organelles to be discovered and observed in detail. It was discovered in 1898 by Italian physician Camillo Golgi during an investigation of the nervous system. After first observing it under his ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frederick Bettelheim
Frederick may refer to: People * Frederick (given name), the name Nobility Anhalt-Harzgerode * Frederick, Prince of Anhalt-Harzgerode (1613–1670) Austria * Frederick I, Duke of Austria (Babenberg), Duke of Austria from 1195 to 1198 * Frederick II, Duke of Austria (1219–1246), last Duke of Austria from the Babenberg dynasty * Frederick the Fair (Frederick I of Austria (Habsburg), 1286–1330), Duke of Austria and King of the Romans Baden * Frederick I, Grand Duke of Baden (1826–1907), Grand Duke of Baden * Frederick II, Grand Duke of Baden (1857–1928), Grand Duke of Baden Bohemia * Frederick, Duke of Bohemia (died 1189), Duke of Olomouc and Bohemia Britain * Frederick, Prince of Wales (1707–1751), eldest son of King George II of Great Britain Brandenburg/Prussia * Frederick I, Elector of Brandenburg (1371–1440), also known as Frederick VI, Burgrave of Nuremberg * Frederick II, Elector of Brandenburg (1413–1470), Margrave of Brandenburg * Frederick Willia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fibrinopeptide B
The fibrinopeptides, fibrinopeptide A (FpA) and fibrinopeptide B (FpB), are peptides which are located in the central region of the fibrous glycoprotein fibrinogen (factor I) and are cleaved by the enzyme thrombin (factor IIa) to convert fibrinogen into covalently-linked fibrin (factor IA) monomers. The N-terminal FpA is cleaved from the Aα chains of fibrinogen and FpB from the Bβ chains of fibrinogen, with FpA released before FpB. Subsequent to their formation, fibrin monomers are converted to cross-linked fibrin polymers by the action of thrombin-activated factor XIII (fibrin stabilizing factor), and these fibrin polymers form the backbone of a thrombus (blood clot). Hence, the fibrinopeptides are sensitive markers of fibrinogenesis (fibrin generation), thrombin activity, and coagulation. FpA is a 16-amino acid peptide. The half-life of FpA is very short at approximately 3 to 5 minutes. Hence, FpA levels provide a relatively transient measure of coagulation activati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prokaryote
A prokaryote () is a single-celled organism that lacks a nucleus and other membrane-bound organelles. The word ''prokaryote'' comes from the Greek πρό (, 'before') and κάρυον (, 'nut' or 'kernel').Campbell, N. "Biology:Concepts & Connections". Pearson Education. San Francisco: 2003. In the two-empire system arising from the work of Édouard Chatton, prokaryotes were classified within the empire Prokaryota. But in the three-domain system, based upon molecular analysis, prokaryotes are divided into two domains: ''Bacteria'' (formerly Eubacteria) and ''Archaea'' (formerly Archaebacteria). Organisms with nuclei are placed in a third domain, Eukaryota. In the study of the origins of life, prokaryotes are thought to have arisen before eukaryotes. Besides the absence of a nucleus, prokaryotes also lack mitochondria, or most of the other membrane-bound organelles that characterize the eukaryotic cell. It was once thought that prokaryotic cellular components within the cytop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine Protease Inhibitor
Serpins are a superfamily of proteins with similar structures that were first identified for their protease inhibition activity and are found in all kingdoms of life. The acronym serpin was originally coined because the first serpins to be identified act on chymotrypsin-like serine proteases (serine protease inhibitors). They are notable for their unusual mechanism of action, in which they irreversibly inhibit their target protease by undergoing a large conformational change to disrupt the target's active site. This contrasts with the more common competitive mechanism for protease inhibitors that bind to and block access to the protease active site. Protease inhibition by serpins controls an array of biological processes, including coagulation and inflammation, and consequently these proteins are the target of medical research. Their unique conformational change also makes them of interest to the structural biology and protein folding research communities. The conformatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosylprotein Sulfotransferase
Tyrosylprotein sulfotransferase is an enzyme that catalyzes tyrosine sulfation. Function Tyrosylprotein sulfotransferase is the enzyme that catalyzes the sulfation reaction of protein tyrosines, a post-translational modification of proteins. It utilizes 3'-Phosphoadenosine-5'-phosphosulfate (PAPS) as the sulfonate donor and binds proteins with target tyrosine residues to eventually form the tyrosine O-sulfate ester group and the desulfonated 3’-phosphoadenosine-5’-phosphate (PAP). TPST and tyrosine sulfation is involved in a large number of biological and physiological processes. Tyrosine sulfation has been found to be an important part of the inflammatory process, leukocyte movement and cytosis, viral cell entrance, and other cell-cell and protein-protein interactions. Selection for specific tyrosine residues requires a generally accessible tyrosine residue, and acidic residues within +5 or -5 residues of the target tyrosine. P-selectin glycoprotein ligand-1 (PSG ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PROSITE
PROSITE is a protein database. It consists of entries describing the protein families, domains and functional sites as well as amino acid patterns and profiles in them. These are manually curated by a team of the Swiss Institute of Bioinformatics and tightly integrated into Swiss-Prot protein annotation. PROSITE was created in 1988 by Amos Bairoch, who directed the group for more than 20 years. Since July 2018, the director of PROSITE and Swiss-Prot is Alan Bridge. PROSITE's uses include identifying possible functions of newly discovered proteins and analysis of known proteins for previously undetermined activity. Properties from well-studied genes can be propagated to biologically related organisms, and for different or poorly known genes biochemical functions can be predicted from similarities. PROSITE offers tools for protein sequence analysis and motif detection (see sequence motif, PROSITE patterns). It is part of the ExPASy proteomics analysis servers. The database ProR ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Factor VIII
Factor VIII (FVIII) is an essential blood-clotting protein, also known as anti-hemophilic factor (AHF). In humans, factor VIII is encoded by the ''F8'' gene. Defects in this gene result in hemophilia A, a recessive X-linked coagulation disorder. Factor VIII is produced in liver sinusoidal cells and endothelial cells outside the liver throughout the body. This protein circulates in the bloodstream in an inactive form, bound to another molecule called von Willebrand factor, until an injury that damages blood vessels occurs. In response to injury, coagulation factor VIII is activated and separates from von Willebrand factor. The active protein (sometimes written as coagulation factor VIIIa) interacts with another coagulation factor called factor IX. This interaction sets off a chain of additional chemical reactions that form a blood clot. Factor VIII participates in blood coagulation; it is a cofactor for factor IXa, which, in the presence of Ca2+ and phospholipids, forms a complex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |