|
Sucrase-isomaltase
Oligo-1,6-glucosidase (EC 3.2.1.10, sucrase-isomaltase, SI; systematic name oligosaccharide 6-α-glucohydrolase) is a glucosidase enzyme located on the brush border of the small intestine, which catalyses the following reaction: :Hydrolysis of (1→6)-α-D-glucosidic linkages in some oligosaccharides produced from starch and glycogen by (α-amylase), and in isomaltose It is a dual-function enzyme with two GH31 domains, one serving as the isomaltase, the other as a sucrose alpha-glucosidase. It has preferential expression in the apical membranes of enterocytes. The enzyme’s purpose is to digest dietary carbohydrates such as starch, sucrose and isomaltose. By further processing the broken-down products, energy in the form of ATP can be generated.Berg, J. M. et al. ''Biochemistry'', 7th Ed. W.H. Freeman and Company: New York, 2012. Structure Sucrase-isomaltase consists of two enzymatic subunits: sucrase and isomaltase. The subunits originate from a polypeptide precurs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sucrose Intolerance
Sucrose intolerance or genetic sucrase-isomaltase deficiency (GSID) is the condition in which sucrase-isomaltase, an enzyme needed for proper metabolism of sucrose (sugar) and starch (e.g., grains), is not produced or the enzyme produced is either partially functional or non-functional in the small intestine. All GSID patients lack fully functional sucrase, while the isomaltase activity can vary from minimal functionality to almost normal activity. The presence of residual isomaltase activity may explain why some GSID patients are better able to tolerate starch in their diet than others with GSID. Signs and symptoms *Abdominal cramps and bloating *Diarrhea and constipation *Vomiting *Hypoglycemia and headaches *Poor weight gain and growth *Upper respiratory tract and viral diseases *Anxiety and heart palpitations *Excess gas production Cause Sucrose intolerance can be caused by genetic mutations in which both parents must contain this gene for the child to carry the disease (so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucosidase
Glucosidases are the glycoside hydrolase enzymes categorized under the EC number 3.2.1. Function Alpha-glucosidases are enzymes involved in breaking down complex carbohydrates such as starch and glycogen into their monomers. They catalyze the cleavage of individual glucosyl residues from various glycoconjugates including alpha- or beta-linked polymers of glucose. This enzyme convert complex sugars into simpler ones. Members Different sources include different members in this class. Members marked with a "#" are considered by MeSH A mesh is a barrier made of connected strands of metal, fiber, or other flexible or ductile materials. A mesh is similar to a web or a net in that it has many attached or woven strands. Types * A plastic mesh may be extruded, oriented, exp ... to be glucosidases. Clinical significance Alpha-glucosidases are targeted by alpha-glucosidase inhibitors such as acarbose and miglitol to control diabetes mellitus type 2. See also * DNA gl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
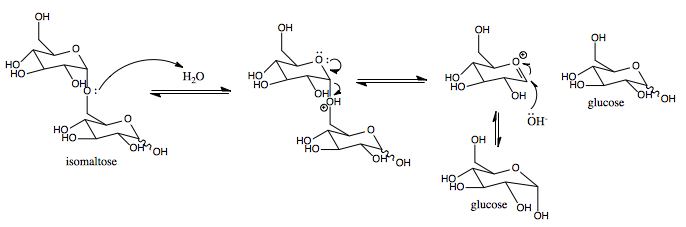
Isomaltase
Isomaltase () is an enzyme that breaks the bonds linking saccharides, which cannot be broken by amylase or maltase. It digests polysaccharides at the alpha 1-6 linkages. Its substrate, alpha-limit dextrin, is a product of amylopectin digestion that retains its 1-6 linkage (its alpha 1-4 linkages having already been broken down by amylase). The product of the enzymatic digestion of alpha-limit dextrin by isomaltase is maltose. Isomaltase helps amylase to digest alpha-limit dextrin to produce maltose. The human sucrase-isomaltase is a dual-function enzyme with two GH31 domains, one serving as the isomaltase, the other as a sucrose alpha-glucosidase. Nomenclature The systematic name of sucrase-isomaltase is oligosaccharide 6-alpha-glucohydrolase. This enzyme is also known as: * Sucrase-alpha-dextrinase * oligo-1,6-glucosidase, * limit dextrin, * so maltase, * exo-oligo-1,6-glucosidase, * dextrin 6alpha-glucanohydrolase, * alpha-limit dextrin, * dextrin 6-glucanohydrolase, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomaltase
Isomaltase () is an enzyme that breaks the bonds linking saccharides, which cannot be broken by amylase or maltase. It digests polysaccharides at the alpha 1-6 linkages. Its substrate, alpha-limit dextrin, is a product of amylopectin digestion that retains its 1-6 linkage (its alpha 1-4 linkages having already been broken down by amylase). The product of the enzymatic digestion of alpha-limit dextrin by isomaltase is maltose. Isomaltase helps amylase to digest alpha-limit dextrin to produce maltose. The human sucrase-isomaltase is a dual-function enzyme with two GH31 domains, one serving as the isomaltase, the other as a sucrose alpha-glucosidase. Nomenclature The systematic name of sucrase-isomaltase is oligosaccharide 6-alpha-glucohydrolase. This enzyme is also known as: * Sucrase-alpha-dextrinase * oligo-1,6-glucosidase, * limit dextrin, * so maltase, * exo-oligo-1,6-glucosidase, * dextrin 6alpha-glucanohydrolase, * alpha-limit dextrin, * dextrin 6-glucanohydrolase, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
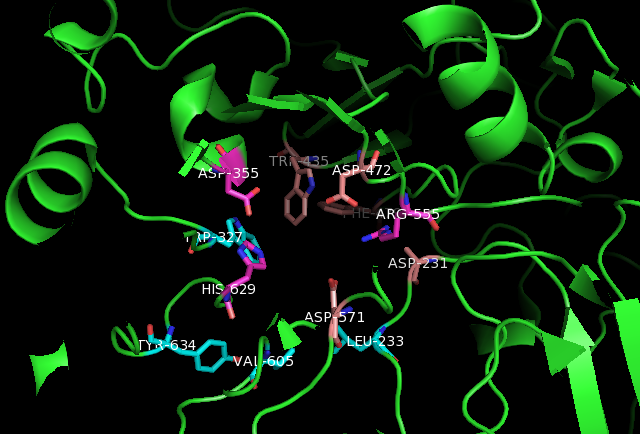
Active Site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate (binding site) and residues that catalyse a reaction of that substrate (catalytic site). Although the active site occupies only ~10–20% of the volume of an enzyme, it is the most important part as it directly catalyzes the chemical reaction. It usually consists of three to four amino acids, while other amino acids within the protein are required to maintain the tertiary structure of the enzymes. Each active site is evolved to be optimised to bind a particular substrate and catalyse a particular reaction, resulting in high specificity. This specificity is determined by the arrangement of amino acids within the active site and the structure of the substrates. Sometimes enzymes also need to bind with some cofactors to fulfil their function. The active si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sucrase
Sucrase is a digestive enzyme that catalyzes the hydrolysis of sucrose to its subunits fructose and glucose. One form, sucrase-isomaltase, is secreted in the small intestine on the brush border. The sucrase enzyme invertase, which occurs more commonly in plants, also hydrolyzes sucrose but by a different mechanism. Types * is isomaltase * is invertase * is sucrose alpha-glucosidase Physiology Sucrose intolerance (also known as congenital sucrase-isomaltase deficiency (CSID), genetic sucrase-isomaltase deficiency (GSID), or sucrase-isomaltase deficiency) occurs when sucrase is not being secreted in the small intestine. With sucrose intolerance, the result of consuming sucrose is excess gas production and often diarrhea and malabsorption. Lactose intolerance is a related disorder that reflects an individual's inability to hydrolyze the disaccharide lactose. Sucrase is secreted by the tips of the villi of the epithelium in the small intestine. Its levels are reduced in response ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chronic Lymphocytic Leukemia
Chronic lymphocytic leukemia (CLL) is a type of cancer in which the bone marrow makes too many lymphocytes (a type of white blood cell). Early on, there are typically no symptoms. Later, non-painful lymph node swelling, feeling tired, fever, night sweats, or weight loss for no clear reason may occur. Enlargement of the spleen and low red blood cells (anemia) may also occur. It typically worsens gradually over years. Risk factors include having a family history of the disease, with 10% of those who develop CLL having a family history of the disease. Exposure to Agent Orange, certain insecticides, sun exposure, exposure to hepatitis C virus, and common infections are also considered risk factors. CLL results in the buildup of B cell lymphocytes in the bone marrow, lymph nodes, and blood. These cells do not function well and crowd out healthy blood cells. CLL is divided into two main types: those with a mutated IGHV gene and those without. Diagnosis is typically based on blood te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acarbose
Acarbose (INN) is an anti-diabetic drug used to treat diabetes mellitus type 2 and, in some countries, prediabetes. It is a generic sold in Europe and China as Glucobay (Bayer AG), in North America as Precose (Bayer Pharmaceuticals), and in Canada as Prandase (Bayer AG). It is cheap and popular in China, but not in the U.S. One physician explains the use in the U.S. is limited because it is not potent enough to justify the side effects of diarrhea and flatulence. However, a recent large study concludes "acarbose is effective, safe and well tolerated in a large cohort of Asian patients with type 2 diabetes." A possible explanation for the differing opinions is an observation that acarbose is significantly more effective in patients eating a relatively high carbohydrate Eastern diet. It is a starch blocker, and inhibits alpha glucosidase, an intestinal enzyme that releases glucose from larger carbohydrates. It is composed of an acarviosin moiety with a maltose at the reducing ter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. History The terms ''nucleophile'' and ''electrophile'' were introduced by Christopher Kelk Ingold in 1933, replacing the terms ''anionoid'' and ''cationoid' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy groups. Both the negatively charged anion , called hydroxide, and the neutral radical , known as the hydroxyl radical, consist of an unbonded hydroxy group. According to IUPAC definitions, the term ''hydroxyl'' refers to the hydroxyl radical () only, while the functional group is called a ''hydroxy group''. Properties Water, alcohols, carboxylic acids, and many other hydroxy-containing compounds can be readily deprotonated due to a large difference between the electronegativity of oxygen (3.5) and that of hydrogen (2.1). Hydroxy-containing compounds engage in intermolecular hydrogen bonding increasing the electrostatic attraction between molecules and thus to higher boiling and melting points than found for compounds that lack this f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bonds
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted , where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are the second-row elements nitrogen (N), oxygen (O), and fluorine (F). Hydrogen bonds can be intermolecular (occurring between separate molecules) or intramolecular (occurring among parts of the same molecule). The energy of a hydrogen bond depends on the geometry, the environment, and the nature of the specific donor and acceptor atoms and can vary between 1 and 40 kcal/mol. This makes them somewhat stronger than a van der Waals interaction, and weaker than fully covalent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maltase-glucoamylase
Maltase-glucoamylase, intestinal is an enzyme that in humans is encoded by the ''MGAM'' gene. Maltase-glucoamylase is an alpha-glucosidase digestive enzyme. It consists of two subunits with differing substrate specificity. Recombinant enzyme studies have shown that its N-terminal catalytic domain has highest activity against maltose, while the C-terminal domain has a broader substrate specificity and activity against glucose oligomers. In the small intestine, this enzyme works in synergy with sucrase-isomaltase and alpha-amylase to digest the full range of dietary starches. Gene The MGAM gene –– which is located on chromosome 7q34 –– codes for the protein Maltase-Glucoamylase. An alternative name for Maltase-Glucoamylase is glucan 1,4-alpha-glycosidase. Tissue distribution Maltase-glucoamylase is a membrane-bound enzyme located in the intestinal walls. This lining of the intestine forms brush border in which food has to pass in order for the intestines to absor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |