|
Sterculic Acid
Sterculic acid is a cyclopropene fatty acid. It is found in various plants of the genus ''Sterculia'', including being the main component of ''Sterculia foetida'' seed oil. Biosynthesis The biosynthesis of sterculic acid begins with the cyclopropanation of the alkene of phospholipid-bound oleic acid, an 18-carbon ''Cis–trans isomerism, cis''-monounsaturated fatty acid. This transformation involves two mechanistic steps: electrophilic methylation with S-Adenosyl methionine, ''S''-adenosyl methionine to give a carbocationic reactive intermediate, followed by cyclization via loss of Hydron (chemistry), H+ mediated by a cyclopropane-fatty-acyl-phospholipid synthase enzyme. The product, dihydrosterculic acid, is converted to sterculic acid by dehydrogenation of the ''cis''-disubstituted cyclopropane to cyclopropene. An additional step of alpha oxidation, α oxidation removes one carbon from the carboxy chain to form the 17-carbon-chain structure of malvalic acid. References F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropene Fatty Acid
Cyclopropane fatty acids (CPA) are a subgroup of fatty acids that contain a cyclopropane group. Although they are usually rare, the seed oil from lychee contains nearly 40% CPAs in the form of triglycerides. Biosynthesis CPAs are derived from unsaturated fatty acids by cyclopropanation. The methylene donor is a methyl group on S-adenosylmethionine (SAM). The conversion is catalyzed by cyclopropane-fatty-acyl-phospholipid synthase. The mechanism is proposed to involve transfer of a CH3+ group from SAM to the alkene, followed by deprotonation of the newly attached methyl group and ring closure. Cyclopropene fatty acids Cyclopropene, Cycloprop''e''ne fatty acids are even rarer than CPAs. The best-known examples are malvalic acid and sterculic acid. Sterculic acid as its triglyceride is present in sterculia oils and at low levels in Kapok tree, kapok seed oil (~12%), cottonseed oil (~1%), and in the seeds of the tree ''Sterculia foetida'' (~65-78%). These acids are highly react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountered (e.g., ethylene dication ). Until the early 1970s, all carbocations were called ''carbonium ions''. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon: three in the carbenium ions and five in the carbonium ions. This nomenclature was proposed by G. A. Olah. Carbonium ions, as originally defined by Olah, are characterized by a three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called 'non-classical carbocations', which are carbocations that contain bridging C–C or C–H σ-bonds. Howe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malvalic Acid
Malvalic acid is a cyclopropene fatty acid found in baobab seed oil and cottonseed oil. The cyclopropene ring is thought to be one of the causes of abnormalities that develop in animals that ingest cottonseed oil. Refining processes, such as hydrogenation, can remove or destroy malvalic acid. Biosynthesis The biosynthesis of malvalic acid starts with oleic acid, an 18-carbon monounsaturated fatty acid, leading to sterculic acid. An α-oxidation reaction removes one carbon from the chain to form the 17-carbon-chain structure of malvalic acid. History Wilson et al. demonstrated the co-occurrence of malvalic acid and the corresponding cyclopropane acids in several types of seeds. He suggested that methylene addition to oleic acid gave rise to dihydrosterculic acid, which was desaturated to sterculic acid, and that 8-heptadecenoic acid was similarly the precursor of dihydromalvalic acid and malvalic acid. Smith and Bu'Lock showed that in ''Hibiscus ''Hibiscus'' is a genus of flo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Oxidation
Enzymatic steps of alpha oxidation, 250px Alpha oxidation (α-oxidation) is a process by which certain branched-chain fatty acids are broken down by removal of a single carbon from the carboxyl end. In humans, alpha-oxidation is used in peroxisomes to break down dietary phytanic acid, which cannot undergo beta-oxidation due to its β-methyl branch, into pristanic acid. Pristanic acid can then acquire acetyl-CoA and subsequently become beta oxidized, yielding propionyl-CoA. Pathway Alpha-oxidation of phytanic acid is believed to take place entirely within peroxisomes. #Phytanic acid is first attached to CoA to form phytanoyl-CoA. # Phytanoyl-CoA is oxidized by phytanoyl-CoA dioxygenase, in a process using Fe2+ and O2, to yield 2-hydroxyphytanoyl-CoA. #2-hydroxyphytanoyl-CoA is cleaved by 2-hydroxyphytanoyl-CoA lyase in a TPP-dependent reaction to form pristanal and formyl-CoA (in turn later broken down into formate and eventually CO2). #Pristanal is oxidized by aldehyde dehydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropene
Cyclopropene is an organic compound with the formula . It is the simplest cycloalkene. Because the ring is highly strained, cyclopropene is difficult to prepare and highly reactive. This colorless gas has been the subject for many fundamental studies of bonding and reactivity. It does not occur naturally, but derivatives are known in some fatty acids. Derivatives of cyclopropene are used commercially to control ripening of some fruit. Structure and bonding The molecule has a triangular structure. The reduced length of the double bond compared to a single bond causes the angle opposite the double bond to narrow to about 51° from the 60° angle found in cyclopropane. As with cyclopropane, the carbon–carbon bonding in the ring has increased p character: the alkene carbon atoms use sp2.68 hybridization for the ring. Synthesis of cyclopropene and derivatives Early syntheses The first confirmed synthesis of cyclopropene, carried out by Dem'yanov and Doyarenko, involved the the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself is mainly of theoretical interest but many of its derivatives are of commercial or biological significance. History Cyclopropane was discovered in 1881 by August Freund, who also proposed the correct structure for the substance in his first paper. Freund treated 1,3-dibromopropane with sodium, causing an intramolecular Wurtz reaction leading directly to cyclopropane. The yield of the reaction was improved by Gustavson in 1887 with the use of zinc instead of sodium. Cyclopropane had no commercial application until Henderson and Lucas discovered its anaesthetic properties in 1929; industrial production had begun by 1936. In modern anaesthetic practice, it has been superseded by other agents. Anaesthesia Cyclopropane was introduced into cli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydrogenation
In chemistry, dehydrogenation is a chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem. At its simplest, it is useful way of converting alkanes, which are relatively inert and thus low-valued, to olefins, which are reactive and thus more valuable. Alkenes are precursors to aldehydes (), alcohols (), polymers, and aromatics. As a problematic reaction, the fouling and inactivation of many catalysts arises via coking, which is the dehydrogenative polymerization of organic substrates. Enzymes that catalyze dehydrogenation are called dehydrogenases. Heterogeneous catalytic routes Styrene Dehydrogenation processes are used extensively to produce aromatics in the petrochemical industry. Such processes are highly endothermic and require temperatures of 500 °C and above. Dehydrogenation also converts saturated fats to unsatura ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropane-fatty-acyl-phospholipid Synthase
In enzymology, a cyclopropane-fatty-acyl-phospholipid synthase () is an enzyme that catalyzes the chemical reaction :S-adenosyl-L-methionine + phospholipid olefinic fatty acid \rightleftharpoons S-adenosyl-L-homocysteine + phospholipid cyclopropane fatty acid Thus, the two substrates of this enzyme are S-adenosyl methionine and phospholipid olefinic fatty acid, whereas its two products are S-adenosylhomocysteine and phospholipid cyclopropane fatty acid. This enzyme belongs to the family of transferases, specifically those transferring one-carbon group methyltransferases. The systematic name of this enzyme class is S-adenosyl-L-methionine:unsaturated-phospholipid methyltransferase (cyclizing). Other names in common use include cyclopropane synthetase, unsaturated-phospholipid methyltransferase, cyclopropane synthase, cyclopropane fatty acid synthase, cyclopropane fatty acid synthetase, and CFA synthase. Structural studies As of late 2007, 6 structures A structure is an ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydron (chemistry)
In chemistry, the hydron, informally called proton, is the cationic form of atomic hydrogen, represented with the symbol . The general term "hydron", endorsed by the IUPAC, encompasses cations of hydrogen regardless of their isotopic composition: thus it refers collectively to protons (1H+) for the protium isotope, deuterons (2H+ or D+) for the deuterium isotope, and tritons (3H+ or T+) for the tritium isotope. Unlike most other ions, the hydron consists only of a bare atomic nucleus. The negatively charged counterpart of the hydron is the hydride anion, . Properties Solute properties Other things being equal, compounds that readily donate hydrons (Brønsted acids, see below) are generally polar, hydrophilic solutes and are often soluble in solvents with high relative static permittivity (dielectric constants). Examples include organic acids like acetic acid (CH3COOH) or methanesulfonic acid (CH3SO3H). However, large nonpolar portions of the molecule may attenuate these pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation. When their existence is indicated, reactive intermediates can help explain how a chemical reaction takes place. Most chemical reactions take more than one elementary step to complete, and a reactive intermediate is a high-energy, yet stable, product that exists only in one of the intermediate steps. The series of steps together make a reaction mechanism. A reactive intermediate differs from a reactant or product or a simple reaction intermediate only in that it cannot usually be isolated but is sometimes observable only through fast spectroscopic methods. It is stable in the sense that an elementary reaction forms the reactive intermediate and the elementary reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
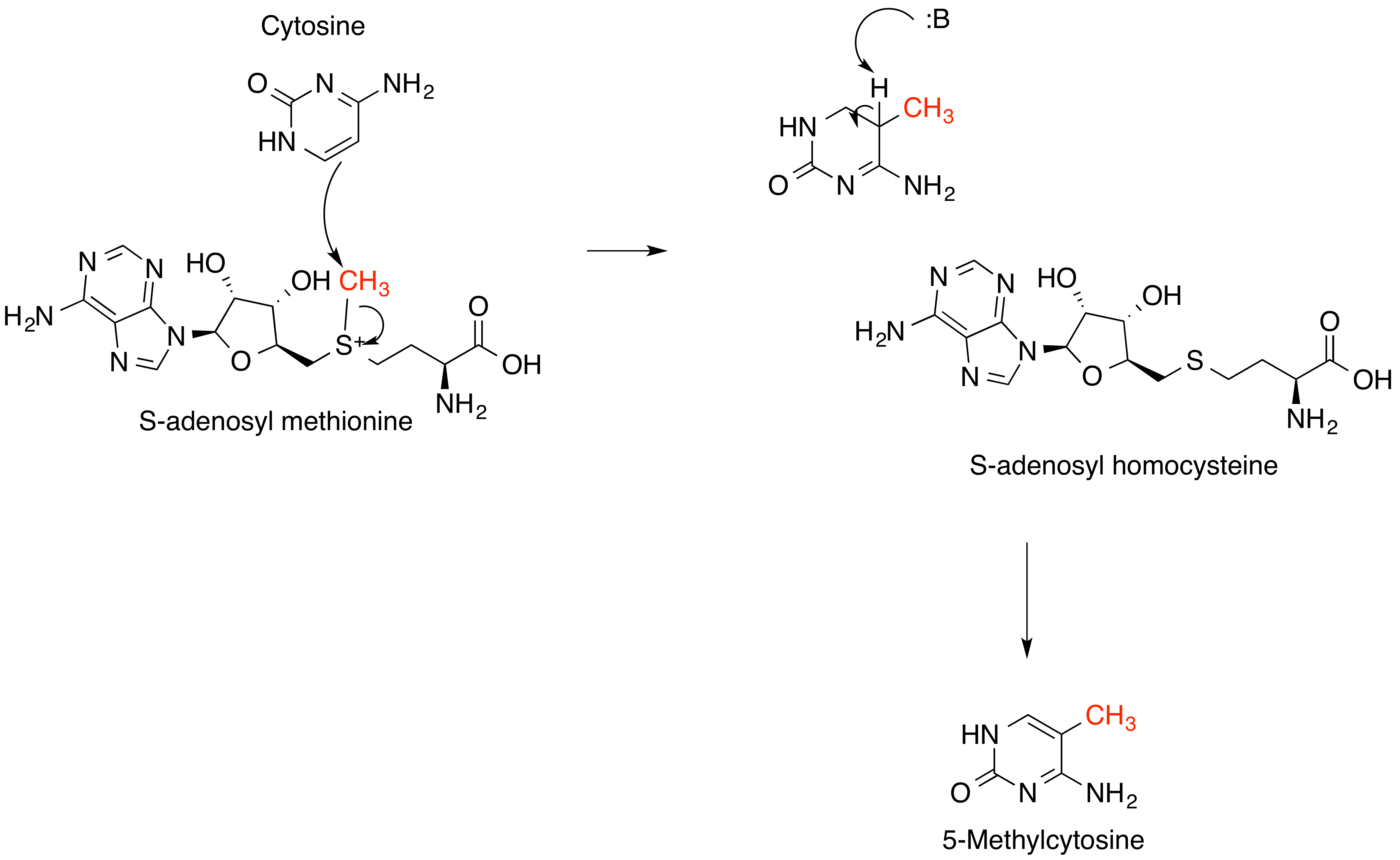
S-Adenosyl Methionine
''S''-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver. More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952. In bacteria, SAM is bound by the SAM riboswitch, which regulates genes involved in methionine or cysteine biosynthesis. In eukaryotic cells, SAM serves as a regulator of a variety of processes including DNA, tRNA, and rRNA methylation; immune response; amino acid metabolism; transsulfuration; and more. In plants, SAM is crucial to the biosynthesis of ethylene, an important plant hormone and sig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sterculia
''Sterculia'' is a genus of flowering plants in the mallow family, Malvaceae: subfamily Sterculioideae (previously placed in the now obsolete Sterculiaceae). Members of the genus are colloquially known as tropical chestnuts. ''Sterculia'' may be monoecious or dioecious, and its flowers unisexual or bisexual. Taxonomy Phylogeny A 27-million-year-old †''Sterculia labrusca'' leaf fossil is described from the Evros region in Western Thrace, Greece. Species The Plant List counts 91 currently accepted species. The accepted species are listed here, except as noted. *''Sterculia abbreviata'' E.L.Taylor ex Mondragón *''Sterculia aerisperma'' Cuatrec. *''Sterculia africana'' ( Lour.) Fiori – Mopopaja tree *''Sterculia albidiflora'' Ducke *''Sterculia alexandri'' Harv. – Cape sterculia *''Sterculia amazonica'' E.L.Taylor ex Mondragón *''Sterculia antioquia'' E.L.Taylor *''Sterculia apeibophylla'' Ducke *''Sterculia alexandri'' ( Jacq.) H.Karst. *''Sterculia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


_(8679258490).jpg)