|
Silicon-based Life
Hypothetical types of biochemistry are forms of biochemistry agreed to be scientifically viable but not proven to exist at this time. The kinds of living organisms currently known on Earth all use carbon compounds for basic structural and metabolic functions, water as a solvent, and DNA or RNA to define and control their form. If life exists on other planets or moons it may be chemically similar, though it is also possible that there are organisms with quite different chemistries for instance, involving other classes of carbon compounds, compounds of another element, or another solvent in place of water. The possibility of life-forms being based on "alternative" biochemistries is the topic of an ongoing scientific discussion, informed by what is known about extraterrestrial environments and about the chemical behaviour of various elements and compounds. It is of interest in synthetic biology and is also a common subject in science fiction. The element silicon has been much d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or exemplified by the odors of gasoline and lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to the naturally occurring petroleum, natural gas and coal, and to their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are carbon di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenic
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, but only the gray form, which has a metallic appearance, is important to industry. The primary use of arsenic is in alloys of lead (for example, in car batteries and ammunition). Arsenic is a common n-type dopant in semiconductor electronic devices. It is also a component of the III-V compound semiconductor gallium arsenide. Arsenic and its compounds, especially the trioxide, are used in the production of pesticides, treated wood products, herbicides, and insecticides. These applications are declining with the increasing recognition of the toxicity of arsenic and its compounds. A few species of bacteria are able to use arsenic compounds as respiratory metabolites. Trace quantities of arsenic are an essential dietary element in rats, ham ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenic Biochemistry
Arsenic biochemistry refers to biochemical processes that can use arsenic or its compounds, such as arsenate. Arsenic is a moderately abundant element in Earth's crust, and although many arsenic compounds are often considered highly toxic to most life, a wide variety of organoarsenic compounds are produced biologically and various organic and inorganic arsenic compounds are metabolized by numerous organisms. This pattern is general for other related elements, including selenium, which can exhibit both beneficial and deleterious effects. Arsenic biochemistry has become topical since many toxic arsenic compounds are found in some aquifers, potentially affecting many millions of people via biochemical processes.Elke Dopp, Andrew D. Kligerman and Roland A. Diaz-Bone Organoarsenicals. Uptake, Metabolism, and Toxicity 2010, Royal Society of Chemistry. . Sources of arsenic Organoarsenic compounds in nature The evidence that arsenic may be a beneficial nutrient at trace levels below th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both causti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both caust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epimerase And Racemase
Epimerases and racemases are isomerase enzymes that catalyze the inversion of stereochemistry in biological molecules. Racemases catalyze the stereochemical inversion around the asymmetric carbon atom in a substrate having only one center of asymmetry. Epimerases catalyze the stereochemical inversion of the configuration about an asymmetric carbon atom in a substrate having more than one center of asymmetry, thus interconverting epimers. Human epimerases include methylmalonyl-CoA epimerase, involved in the metabolic breakdown of the amino acids alanine, isoleucine, methionine and valine, and UDP-glucose 4-epimerase, which is used in the final step of galactose metabolism - catalyzing the reversible conversion of UDP-galactose to UDP-glucose. See also * Galactose epimerase deficiency Galactose epimerase deficiency, also known as GALE deficiency, Galactosemia III and UDP-galactose-4-epimerase deficiency, is a rare, autosomal recessive form of galactosemia associated with a defici ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
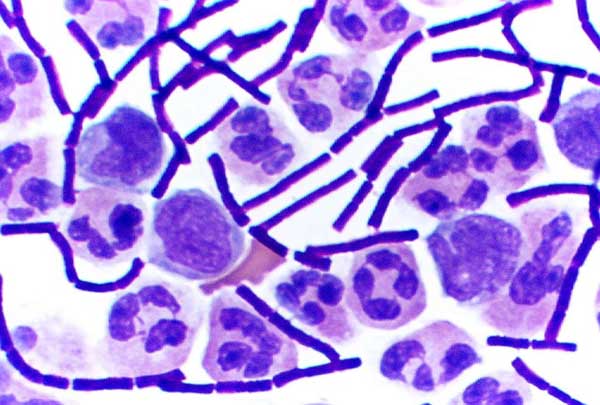
Gram-positive Bacteria
In bacteriology, gram-positive bacteria are bacteria that give a positive result in the Gram stain test, which is traditionally used to quickly classify bacteria into two broad categories according to their type of cell wall. Gram-positive bacteria take up the crystal violet stain used in the test, and then appear to be purple-coloured when seen through an optical microscope. This is because the thick peptidoglycan layer in the bacterial cell wall retains the stain after it is washed away from the rest of the sample, in the decolorization stage of the test. Conversely, gram-negative bacteria cannot retain the violet stain after the decolorization step; alcohol used in this stage degrades the outer membrane of gram-negative cells, making the cell wall more porous and incapable of retaining the crystal violet stain. Their peptidoglycan layer is much thinner and sandwiched between an inner cell membrane and a bacterial outer membrane, causing them to take up the counterstain (sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double sugars, are molecules made of two bonded monosaccharides; common examples are sucrose (glucose + fructose), lactose (glucose + galactose), and maltose (two molecules of glucose). White sugar is a refined form of sucrose. In the body, compound sugars are hydrolysed into simple sugars. Longer chains of monosaccharides (>2) are not regarded as sugars, and are called oligosaccharides or polysaccharides. Starch is a glucose polymer found in plants, the most abundant source of energy in human food. Some other chemical substances, such as glycerol and sugar alcohols, may have a sweet taste, but are not classified as sugar. Sugars are found in the tissues of most plants. Honey and fruits are abundant natural sources of simple sugars. Suc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Left-handed Protein
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality (). The terms are derived from Ancient Greek χείρ (''cheir'') 'hand'; which is the canonical example of an object with this property. A chiral molecule or ion exists in two stereoisomers that are mirror images of each other, called enantiomers; they are often distinguished as either "right-handed" or "left-handed" by their absolute configuration or some other criterion. The two enantiomers have the same chemical properties, except when reacting with other chiral compounds. They also have the same physical properties, except that they often have opposite optical activities. A homogeneous mixture of the two enantiomers in equal parts is said to be racemic, and it usually differs chemically and physically from the pure enantiomers. Chiral molecules will u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chirality
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object. An object or a system is ''chiral'' if it is distinguishable from its mirror image; that is, it cannot be superimposed onto it. Conversely, a mirror image of an ''achiral'' object, such as a sphere, cannot be distinguished from the object. A chiral object and its mirror image are called ''enantiomorphs'' (Greek, "opposite forms") or, when referring to molecules, '' enantiomers''. A non-chiral object is called ''achiral'' (sometimes also ''amphichiral'') and can be superposed on its mirror image. The term was first used by Lord Kelvin in 1893 in the second Robert Boyle Lecture at the Oxford University Junior Scientific Club which was published in 1894: Human hands are perhaps the most recognized example of chirality. The left hand is a non-superimposable mirror image of the right hand; no matter ho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




-3D-balls.png)