|
Saccharification
In chemistry, saccharification is a term for denoting any chemical change wherein a monosaccharide molecule remains intact after becoming unbound from another saccharide. For example, when a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose). Enzymes such as amylases (e.g. in saliva) and glycoside hydrolase (e.g. within the brush border of the small intestine) are able to perform exact saccharification through enzymatic hydrolysis. Through thermolysis, saccharification can also occur as a transient result, among many other possible effects, during caramelization. Supplying more concentrated digestive enzyme Digestive enzymes are a group of enzymes that break down polymeric macromolecules into their smaller building blocks, in order to facilitate their absorption into the cells of the body. Digestive enzymes are found in the digestive tracts of anima ...s in a small intestine model that was design ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a Chemical reaction, reaction with other Chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
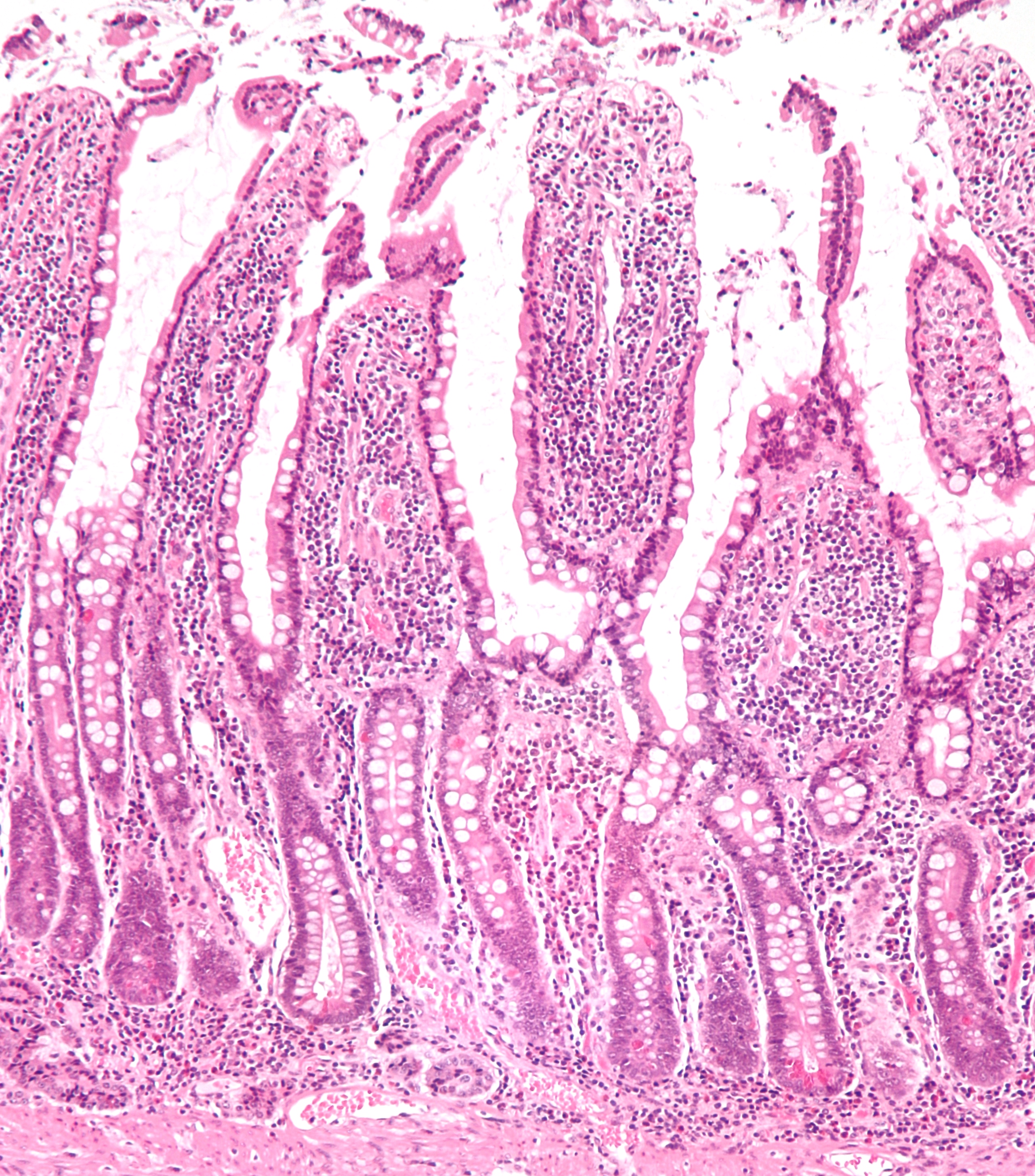
Small Intestine
The small intestine or small bowel is an organ in the gastrointestinal tract where most of the absorption of nutrients from food takes place. It lies between the stomach and large intestine, and receives bile and pancreatic juice through the pancreatic duct to aid in digestion. The small intestine is about long and folds many times to fit in the abdomen. Although it is longer than the large intestine, it is called the small intestine because it is narrower in diameter. The small intestine has three distinct regions – the duodenum, jejunum, and ileum. The duodenum, the shortest, is where preparation for absorption through small finger-like protrusions called villi begins. The jejunum is specialized for the absorption through its lining by enterocytes: small nutrient particles which have been previously digested by enzymes in the duodenum. The main function of the ileum is to absorb vitamin B12, bile salts, and whatever products of digestion that were not absorbed by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gelation
In polymer chemistry, gelation (gel transition) is the formation of a gel from a system with polymers. Branched polymers can form links between the chains, which lead to progressively larger polymers. As the linking continues, larger branched polymers are obtained and at a certain extent of the reaction links between the polymer result in the formation of a single macroscopic molecule. At that point in the reaction, which is defined as gel point, the system loses fluidity and viscosity becomes very large. The onset of gelation, or gel point, is accompanied by a sudden increase in viscosity. This "infinite" sized polymer is called the gel or network, which does not dissolve in the solvent, but can swell in it. Background Gelation is promoted by gelling agents. Gelation can occur either by physical linking or by chemical crosslinking. While the physical gels involve physical bonds, chemical gelation involves covalent bonds. The first quantitative theories of chemical gelation w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoside Hydrolase
Glycoside hydrolases (also called glycosidases or glycosyl hydrolases) catalyze the hydrolysis of glycosidic bonds in complex sugars. They are extremely common enzymes with roles in nature including degradation of biomass such as cellulose (cellulase), hemicellulose, and starch (amylase), in anti-bacterial defense strategies (e.g., lysozyme), in pathogenesis mechanisms (e.g., viral neuraminidases) and in normal cellular function (e.g., trimming mannosidases involved in N-linked glycoprotein biosynthesis). Together with glycosyltransferases, glycosidases form the major catalytic machinery for the synthesis and breakage of glycosidic bonds. Occurrence and importance Glycoside hydrolases are found in essentially all domains of life. In prokaryotes, they are found both as intracellular and extracellular enzymes that are largely involved in nutrient acquisition. One of the important occurrences of glycoside hydrolases in bacteria is the enzyme beta-galactosidase (LacZ), which i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosidic Bond
A glycosidic bond or glycosidic linkage is a type of covalent bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate. A glycosidic bond is formed between the hemiacetal or hemiketal group of a saccharide (or a molecule derived from a saccharide) and the hydroxyl group of some compound such as an alcohol. A substance containing a glycosidic bond is a glycoside. The term 'glycoside' is now extended to also cover compounds with bonds formed between hemiacetal (or hemiketal) groups of sugars and several chemical groups other than hydroxyls, such as -SR (thioglycosides), -SeR (selenoglycosides), -NR1R2 (N-glycosides), or even -CR1R2R3 (C-glycosides). Particularly in naturally occurring glycosides, the compound ROH from which the carbohydrate residue has been removed is often termed the aglycone, and the carbohydrate residue itself is sometimes referred to as the 'glycone'. S-, N-, C-, and O-glycosidic bonds Glycosidi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viscous
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water. Viscosity quantifies the internal frictional force between adjacent layers of fluid that are in relative motion. For instance, when a viscous fluid is forced through a tube, it flows more quickly near the tube's axis than near its walls. Experiments show that some stress (such as a pressure difference between the two ends of the tube) is needed to sustain the flow. This is because a force is required to overcome the friction between the layers of the fluid which are in relative motion. For a tube with a constant rate of flow, the strength of the compensating force is proportional to the fluid's viscosity. In general, viscosity depends on a fluid's state, such as its temperature, pressure, and rate of deformation. However, the dependence on some of these properties is n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Starch
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human diets, and is contained in large amounts in staple foods such as wheat, potatoes, maize (corn), rice, and cassava (manioc). Pure starch is a white, tasteless and odorless powder that is insoluble in cold water or alcohol. It consists of two types of molecules: the linear and helical amylose and the branched amylopectin. Depending on the plant, starch generally contains 20 to 25% amylose and 75 to 80% amylopectin by weight. Glycogen, the energy reserve of animals, is a more highly branched version of amylopectin. In industry, starch is often converted into sugars, for example by malting. These sugars may be fermented to produce ethanol in the manufacture of beer, whisky and biofuel. In addition, sugars produced from processed starch are used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Digestive Enzyme
Digestive enzymes are a group of enzymes that break down polymeric macromolecules into their smaller building blocks, in order to facilitate their absorption into the cells of the body. Digestive enzymes are found in the digestive tracts of animals (including humans) and in the tracts of carnivorous plants, where they aid in the digestion of food, as well as inside cells, especially in their lysosomes, where they function to maintain cellular survival. Digestive enzymes of diverse specificities are found in the saliva secreted by the salivary glands, in the secretions of cells lining the stomach, in the pancreatic juice secreted by pancreatic exocrine cells, and in the secretions of cells lining the small and large intestines. Digestive enzymes are classified based on their target substrates: * Lipases split fatty acids into fats and oils. *Proteases and peptidases split proteins into small peptides and amino acids. *Amylases split carbohydrates such as starch and sugars into s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caramelization
Caramelization is a process of browning of sugar used extensively in cooking for the resulting sweet nutty flavor and brown color. The brown colors are produced by three groups of polymers: caramelans (C24H36O18), caramelens (C36H50O25), and caramelins (C125H188O80). As the process occurs, volatile chemicals such as diacetyl are released, producing the characteristic caramel flavor. Like the Maillard reaction, caramelization is a type of non-enzymatic browning. Unlike the Maillard reaction, caramelization is pyrolytic, as opposed to being a reaction with amino acids. When caramelization involves the disaccharide sucrose, it is broken down into the monosaccharides fructose and glucose. Process Caramelization is a complex, poorly understood process that produces hundreds of chemical products, and includes the following types of reactions: * equilibration of anomeric and ring forms * sucrose inversion to fructose and glucose * condensation reactions * intramolecular bon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Decomposition
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway and possibly an explosion or other chemical reaction. Decomposition temperature definition A simple substance (like water) may exist in equilibrium with its thermal decomposition products, effectively halting the decomposition. The equilibrium fraction of decomposed molecules increases with the temperature. Examples * Calcium carbonate (limestone or chalk) decomposes into calcium oxide and carbon dioxide when heated. The chemical reaction is as follows: ::CaCO3 → CaO + CO2 :The reaction is used to make Calcium oxide, quick lime, which is an industrially impor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzymatic Hydrolysis
In biochemistry, enzymatic hydrolysis is a process in which enzymes facilitate the cleavage of bonds in molecules with the addition of the elements of water (i.e. hydrolysis). It plays an important role in the digestion of food. It may be used to help provide renewable energy, as with cellulosic ethanol.Ethanol from biomass by enzymatic hydrolysis - JD Wright - Chemical Engineering Progress, 1988 osti.gov/ref> See also *Acid hydrolysis *Alkaline hydrolysis *Digestion enzyme Digestive enzymes are a group of enzymes that break down polymeric macromolecules into their smaller building blocks, in order to facilitate their absorption into the cells of the body. Digestive enzymes are found in the digestive tracts of anim ... References Digestive system {{enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |