|
Sulfate Conjugate
Sulfate conjugates are a heterogeneous class of polar, anionic organosulfate compounds containing an ester of sulfuric acid. Sulfate conjugates commonly result from the metabolic conjugation of endogenous and exogenous compounds with sulfate (-OSO3−). Biosynthesis of sulfate esters requires an activated sulfate donor, usually adenosine 5'-phosphosulfate (APS) or 3'-phosphoadenosine-5'-phosphosulfate (PAPS).M. T. Madigan, J. M. Martinko, J. Parker "Brock Biology of Microorganisms" Prentice Hall, 1997. . Sulfate esters may be hydrolyzed by sulfatase enzyme to release the parent alcohol and a sulfate ion.Anderson, CJ, Lucas, LJH, Widlanski, TSMolecular Recognition in Biological Systems: Phosphate Esters vs Sulfate Esters and the Mechanism of Action of Steroid SulfatasesJ. Am. Chem. Soc., 1995, 117 (13), pp 3889–3890 DOI: 10.1021/ja00118a034 Publication Date: April 1995 Steroid sulfation is one of the most common of all forms of steroid conjugation. Except for cholesterol, dehydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Polarity
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end. Polar molecules must contain one or more polar bonds due to a difference in electronegativity between the bonded atoms. Molecules containing polar bonds have no molecular polarity if the bond dipoles cancel each other out by symmetry. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points. Polarity of bonds Not all atoms attract electrons with the same force. The amount of "pull" an atom exerts on its electrons is called its electronegativity. Atoms with high electronegativitiessuch as fluorine, oxygen, and nitrogenexert a greater pull on electrons than atoms with lower electronegativities such as alkali metals and alkaline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmembrane Proteins
A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them (beta-barrels) can be also extracted using denaturing agents. The peptide sequence that spans the membrane, or the transmembrane segment, is largely hydrophobic and can be visualized using the hydropathy plot. Depending on the number of transmembrane segments, transmembrane proteins can be classified as single-span (or bitopic) or multi-span (polytopic). Some other integral membrane proteins are called monotopic, meaning that they are also permanently attached to the membrane, but do not pass t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate Esters
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid. Spelling "Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English. Structure The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry is the same as that of methane. The sulfur atom is in the +6 oxidation state while the four oxygen atoms are each in the −2 state. The sulfate ion carries an overall charge of −2 and it is the conjugate base of the bisulfate (or hydrogensulfate) ion, , which is in turn the conjugate base of , sulfuric acid. Organic sulfate esters, such as dimethyl sulfate, are covalent compounds and esters of sulfuric acid. The tetrahedral molecular geometry of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quercetin 3-O-sulfate
Quercetin 3-sulfate is a plasma human metabolite of quercetin. It is the sulfate conjugate of quercetin. Quercetin-3-sulfate 3'-sulfotransferase is an enzyme that uses 3'-phosphoadenylyl sulfate and quercetin 3-sulfate to produce adenosine 3',5'-bisphosphate and quercetin 3,3'-bissulfate. Quercetin-3-sulfate 4'-sulfotransferase In enzymology, a quercetin-3-sulfate 4'-sulfotransferase () is an enzyme that catalysis, catalyzes the chemical reaction :3'-phosphoadenylyl sulfate + quercetin 3-sulfate \rightleftharpoons adenosine 3',5'-bisphosphate + quercetin 3,4'-bissulfate ... is an enzyme that uses 3'-phosphoadenylyl sulfate and quercetin 3-sulfate to produce adenosine 3',5'-bisphosphate and quercetin 3,4'-bissulfate. Both enzymes can be found in '' Flaveria chlorifolia''. Quercetin-3,3'-bissulfate 7-sulfotransferase is an enzyme that uses 3'-phosphoadenylyl sulfate and quercetin 3,3'-bissulfate to produce adenosine 3',5'-bisphosphate and quercetin 3,7,3'-trissulfate. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estrone Sulfotransferase
Estrone sulfotransferase (EST) (), also known as estrogen sulfotransferase, is an enzyme that catalyzes the transformation of an unconjugated estrogen like estrone into a sulfated estrogen like estrone sulfate. It is a steroid sulfotransferase and belongs to the family of transferases, to be specific, the sulfotransferases, which transfer sulfur-containing groups. This enzyme participates in androgen and estrogen metabolism and sulfur metabolism. Steroid sulfatase is an enzyme that catalyzes the reverse reaction, the transfer of a sulfate to an unconjugated estrogen. Reaction In enzymology, an EST is an enzyme that catalyzes the following chemical reaction: :3'-phosphoadenylyl sulfate + estrone \rightleftharpoons adenosine 3',5'-bisphosphate + estrone 3-sulfate Thus, the two substrates of this enzyme are 3'-phosphoadenylyl sulfate and estrone, whereas its two products are adenosine 3',5'-bisphosphate and estrone 3-sulfate. The enzyme also catalyzes the same reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estrone Sulfate
Estrone sulfate, also known as E1S, E1SO4 and estrone 3-sulfate, is a natural, endogenous steroid and an estrogen ester and conjugate. In addition to its role as a natural hormone, estrone sulfate is used as a medication, for instance in menopausal hormone therapy; for information on estrone sulfate as a medication, see the estrone sulfate (medication) article. Biological function E1S itself is biologically inactive, with less than 1% of the relative binding affinity of estradiol for the ERα and ERβ. However, it can be transformed by steroid sulfatase, also known as estrogen sulfatase, into estrone, an estrogen. Simultaneously, estrogen sulfotransferases, including SULT1A1 and SULT1E1, convert estrone to E1S, resulting in an equilibrium between the two steroids in various tissues. Estrone can also be converted by 17β-hydroxysteroid dehydrogenases into the more potent estrogen estradiol. E1S levels are much higher than those of estrone and estradiol, and it is thought ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydroepiandrosterone Sulfate
Dehydroepiandrosterone sulfate, abbreviated as DHEA sulfate or DHEA-S, also known as androstenolone sulfate, is an endogenous androstane steroid that is produced by the adrenal cortex. It is the 3β-sulfate ester and a metabolite of dehydroepiandrosterone (DHEA) and circulates in far greater relative concentrations than DHEA. The steroid is hormonally inert and is instead an important neurosteroid and neurotrophin. Biological activity Neurosteroid activity Similarly to other conjugated steroids, DHEA-S is devoid of hormonal activity, lacking affinity for the steroid hormone receptors. However, DHEA-S retains activity as a neurosteroid and neurotrophin. It has been found to act as a positive allosteric modulator of the NMDA receptor (50 nM–1 μM), negative allosteric modulator of the GABAA and glycine receptors, and weak agonist of the sigma-1 receptor (Kd > 50 μM). In addition, DHEA-S has been found to directly bind to and activate the TrkA and p75NT ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate Ion
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid. Spelling "Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English. Structure The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry is the same as that of methane. The sulfur atom is in the +6 oxidation state while the four oxygen atoms are each in the −2 state. The sulfate ion carries an overall charge of −2 and it is the conjugate base of the bisulfate (or hydrogensulfate) ion, , which is in turn the conjugate base of , sulfuric acid. Organic sulfate esters, such as dimethyl sulfate, are covalent compounds and esters of sulfuric acid. The tetrahedral molecular geometry of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
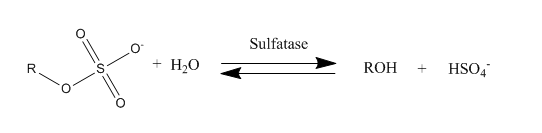
Sulfatase
Sulfatases are enzymes of the esterase class that catalyze the hydrolysis of sulfate esters. These may be found on a range of substrates, including steroids, carbohydrates and proteins. Sulfate esters may be formed from various alcohols and amines. In the latter case the resultant N-sulfates can also be termed sulfamates. Sulfatases play important roles in the cycling of sulfur in the environment, in the degradation of sulfated glycosaminoglycans and glycolipids in the lysosome, and in remodelling sulfated glycosaminoglycans in the extracellular space. Together with sulfotransferases, sulfatases form the major catalytic machinery for the synthesis and breakage of sulfate esters. Occurrence and importance Sulfatases are found in lower and higher organisms. In higher organisms they are found in intracellular and extracellular spaces. Steroid sulfatase is distributed in a wide range of tissues throughout the body, enabling sulfated steroids synthesized in the adrenals and gonads ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




.jpg)