|
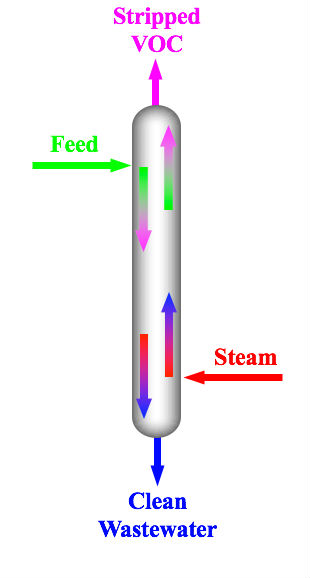
Steam Stripping
Steam stripping is a process used in petroleum refineries and petrochemical plants to remove volatile contaminants, such as hydrocarbons and other volatile organic compounds (VOCs), from wastewater.Beychok, M.R., ''The Design of Sour Water Strippers'', Individual Paper 61, Proceedings of Seventh World Petroleum Congress, Mexico City, April 1967 It typically consists of passing a stream of superheated steam through the wastewater. This method is effective when the volatile compounds have lower boiling points than water or have limited solubility In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubil ... in water. References Petroleum engineering Petrochemical industry Industrial emissions control {{environment-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steam Distillation Diagram
Steam is a substance containing water in the gas phase, and sometimes also an aerosol of liquid water droplets, or air. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization. Steam that is saturated or superheated is invisible; however, "steam" often refers to wet steam, the visible mist or aerosol of water droplets formed as water vapor condenses. Water increases in volume by 1,700 times at standard temperature and pressure; this change in volume can be converted into mechanical work by steam engines such as reciprocating piston type engines and steam turbines, which are a sub-group of steam engines. Piston type steam engines played a central role in the Industrial Revolution and modern steam turbines are used to generate more than 80% of the world's electricity. If liquid water comes in contact with a very hot surface or depressurizes quickly below its vapor pressure, it can create a steam explosion. Types ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oil Refinery
An oil refinery or petroleum refinery is an industrial process plant where petroleum (crude oil) is transformed and refined into useful products such as gasoline (petrol), diesel fuel, asphalt base, fuel oils, heating oil, kerosene, liquefied petroleum gas and petroleum naphtha. Petrochemicals feedstock like ethylene and propylene can also be produced directly by cracking crude oil without the need of using refined products of crude oil such as naphtha. The crude oil feedstock has typically been processed by an oil production plant. There is usually an oil depot at or near an oil refinery for the storage of incoming crude oil feedstock as well as bulk liquid products. In 2020, the total capacity of global refineries for crude oil was about 101.2 million barrels per day. Oil refineries are typically large, sprawling industrial complexes with extensive piping running throughout, carrying streams of fluids between large chemical processing units, such as distillation colu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Petrochemical
Petrochemicals (sometimes abbreviated as petchems) are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane. The two most common petrochemical classes are olefins (including ethylene and propylene) and aromatics (including benzene, toluene and xylene isomers). Oil refineries produce olefins and aromatics by fluid catalytic cracking of petroleum fractions. Chemical plants produce olefins by steam cracking of natural gas liquids like ethane and propane. Aromatics are produced by catalytic reforming of naphtha. Olefins and aromatics are the building-blocks for a wide range of materials such as solvents, detergents, and adhesives. Olefins are the basis for polymers and oligomers used in plastics, resins, fibers, elastomers, lubricants, and gels. Global ethylene production was 190 million tonnes an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volatiles
Volatiles are the group of chemical elements and chemical compounds that can be readily vaporized. In contrast with volatiles, elements and compounds that are not readily vaporized are known as refractory substances. On planet Earth, the term 'volatiles' often refers to the volatile components of magma. In astrogeology volatiles are investigated in the crust or atmosphere of a planet or moon. Volatiles include nitrogen, carbon dioxide, ammonia, hydrogen, methane, sulfur dioxide, water and others. Planetary science Planetary scientists often classify volatiles with exceptionally low melting points, such as hydrogen and helium, as gases (as in gas giant), whereas those volatiles with melting points above about 100 K (–173 °C, –280 °F) are referred to as ices. The terms "gas" and "ice" in this context can apply to compounds that may be solids, liquids or gases. Thus, Jupiter and Saturn are gas giants, and Uranus and Neptune are ice giants, even though the vast majori ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or exemplified by the odors of gasoline and lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to the naturally occurring petroleum, natural gas and coal, and to their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are carbon di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volatile Organic Compounds
Volatile organic compounds (VOCs) are organic compounds that have a high vapour pressure at room temperature Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on .... High vapor pressure correlates with a low boiling point, which relates to the number of the sample's molecules in the surrounding air, a trait known as volatility (chemistry), volatility. VOCs are responsible for the odor of scents and perfumes as well as pollutants. VOCs play an important role in communication between animals and plants, e.g. attractants for pollinators, protection from predation, and even inter-plant interactions. Some VOCs are dangerous to human health or cause harm to the natural environment, environment. Human impact on the environment, Anthropogenic VOCs are regulated by law, especially indoors, where c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wastewater
Wastewater is water generated after the use of freshwater, raw water, drinking water or saline water in a variety of deliberate applications or processes. Another definition of wastewater is "Used water from any combination of domestic, industrial, commercial or agricultural activities, surface runoff / storm water, and any sewer inflow or sewer infiltration". In everyday usage, wastewater is commonly a synonym for sewage (also called sewerage, domestic wastewater, or municipal wastewater), which is wastewater that is produced by a community of people. As a generic term wastewater may also be used to describe water containing contaminants accumulated in other settings, such as: * Industrial wastewater: waterborne waste generated from a variety of industrial processes, such as manufacturing operations, mineral extraction, power generation, or water and wastewater treatment. ** Cooling water, released with potential thermal pollution after use to condense steam or reduce machi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distillation Design
''Distillation Design'' is a book which provides complete coverage of the design of industrial distillation columns for the petroleum refining, chemical and petrochemical plants, natural gas processing, pharmaceutical, food and alcohol distilling industries. It has been a classical chemical engineering textbook since it was first published in February 1992. The subjects covered in the book include: *''Vapor–liquid equilibrium(VLE)'': Vapor–liquid K values, relative volatilities, ideal and non-ideal systems, phase diagrams, calculating bubble points and dew points *''Key fractional distillation concepts'': theoretical stages, x-y diagrams, multicomponent distillation, column composition and temperature profiles *''Process design and optimization'': minimum reflux and minimum stages, optimum reflux, short-cut methods, feed entry location *''Rigorous calculation methods'': Bubble point method, sum rates method, numerical methods (Newton–Raphson technique), inside out metho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superheated Steam
Superheated steam is steam at a temperature higher than its vaporization point at the absolute pressure where the temperature is measured. Superheated steam can therefore cool (lose internal energy) by some amount, resulting in a lowering of its temperature without changing state (i.e., condensing) from a gas, to a mixture of saturated vapor and liquid. If unsaturated steam (a mixture which contains both water vapor and liquid water droplets) is heated at constant pressure, its temperature will also remain constant as the vapor quality (think dryness, or percent saturated vapor) increases towards 100%, and becomes dry (i.e., no saturated liquid) saturated steam. Continued heat input will then "super" heat the dry saturated steam. This will occur if saturated steam contacts a surface with a higher temperature. Superheated steam and liquid water cannot coexist under thermodynamic equilibrium, as any additional heat simply evaporates more water and the steam will become saturated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boiling Point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point of a liquid varies depending upon the surrounding environmental pressure. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. A liquid at low pressure has a lower boiling point than when that liquid is at atmospheric pressure. Because of this, water boils at under standard pressure at sea level, but at at altitude. For a given pressure, different liquids will boiling, boil at different temperatures. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals the defined atmospheric pressure at sea level, one Atmosphere (unit), atmosphere. At that temperature, the vapor pressure of the liquid becomes suffici ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be " miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. M. Diepen (1966): "Gas—Gas Equilibria". ''Journal of Chemical Physics'', ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |