|
Sodium Stannite
The stannite ion is , or similar. Stannite ion can be formed by adding strong base to stannous hydroxide. Stannite ion is a strong reducing agent; also, it may Disproportionation, disproportionate to tin metal plus stannate ion. There are stannite compounds, for example, sodium stannite, . See also * Stannate Oxyanions {{Chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stannous Hydroxide
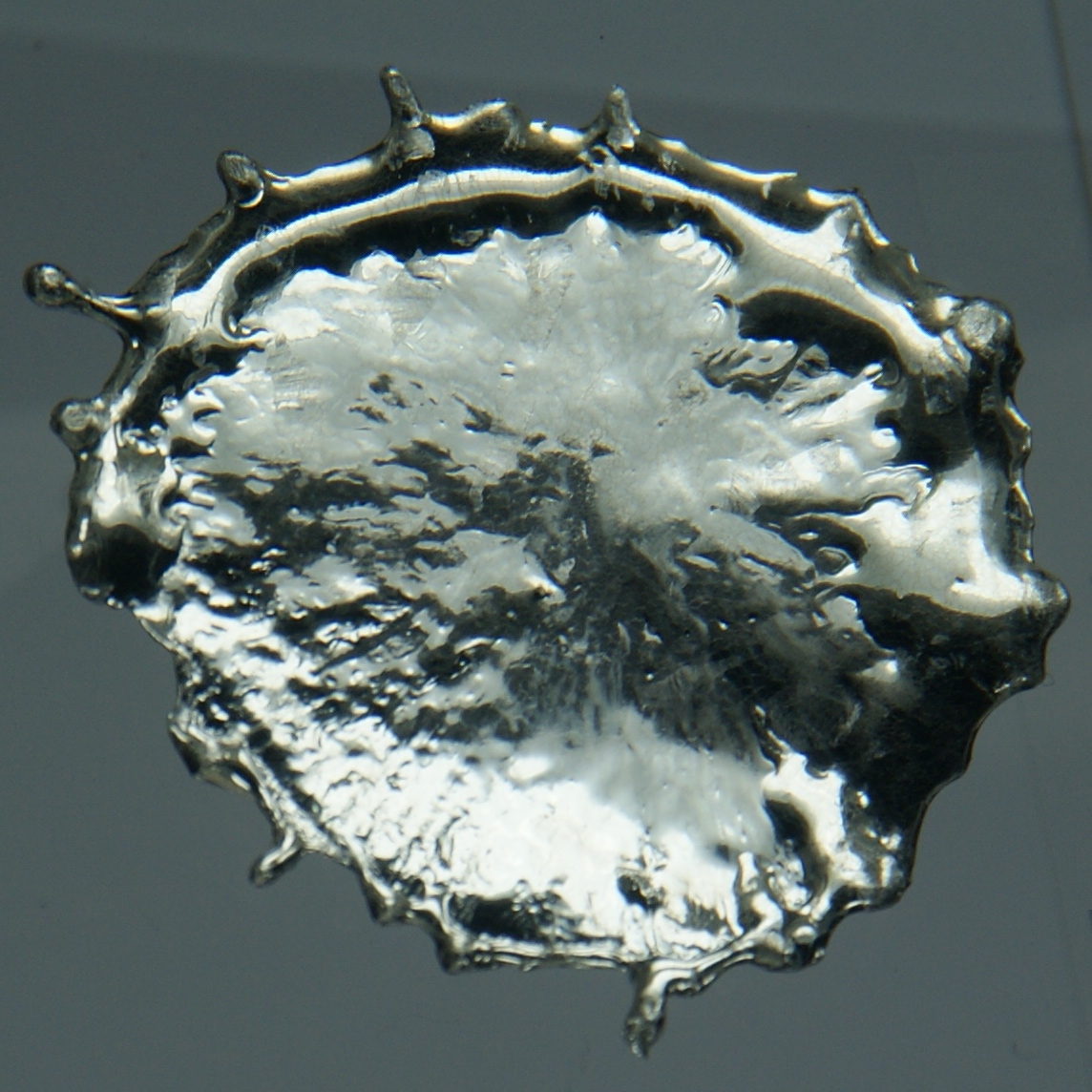
Tin is a chemical element with the Chemical symbol, symbol Sn (from la, :la:Stannum, stannum) and atomic number 50. Tin is a silvery-coloured metal. Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, the so-called "tin cry" can be heard as a result of Crystal twinning, twinning in tin crystals; this trait is shared by indium, cadmium, zinc, and Mercury (element), mercury in the solid state. Pure tin after solidifying presents a mirror-like appearance similar to most metals. In most tin alloys (such as pewter) the metal solidifies with a dull gray color. Tin is a post-transition metal in Carbon group, group 14 of the Periodic table, periodic table of elements. It is obtained chiefly from the mineral cassiterite, which contains Tin(IV) oxide, stannic oxide, . Tin shows a chemical similarity to both of its neighbors in group 14, germanium and lead, and has two main oxidation states, +2 and the slightly more st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reducing Agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ). Examples of substances that are commonly reducing agents include the Earth metals, formic acid, oxalic acid, and sulfite compounds. In their pre-reaction states, reducers have extra electrons (that is, they are by themselves reduced) and oxidizers lack electrons (that is, they are by themselves oxidized). This is commonly expressed in terms of their oxidation states. An agent's oxidation state describes its degree of loss of electrons, where the higher the oxidation state then the fewer electrons it has. So initially, prior to the reaction, a reducing agent is typically in one of its lower possible oxidation states; its oxidation state increases during the reaction while that of the oxidizer decreases. Thus in a redox reaction, the agent whose oxidation state increases, that "loses/Electron donor, donates electrons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. More generally, the term can be applied to any desymmetrizing reaction of the following type, regardless of whether it is a redox or some other type of process: :2A -> A' + A'' Examples *Mercury(I) chloride disproportionates upon UV-irradiation: :Hg2Cl2 → Hg + HgCl2 *Phosphorous acid disproportionates upon heating to give phosphoric acid and phosphine: :4 → 3 H3PO4 + PH3 *Desymmetrizing reactions are sometimes referred to as disproportionation, as illustrated by the thermal degradation of bicarbonate: :2 → + H2CO3 :The oxidation numbers remain constant in this acid-base reaction. This process is also called autoionization. *Another variant on disproportionation is radical disproportionation, in which two radicals form an alkene and an alkane. : Reverse r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stannate
In chemistry the term stannate refers to compounds of tin (Sn). Stannic acid (Sn(OH)4), the formal precursor to stannates, does not exist and is actually a hydrate of SnO2. The term is also used in naming conventions as a suffix; for example the hexachlorostannate ion is . In materials science, two kinds of tin oxyanions are distinguished: *''orthostannates'' contain discrete units (e.g. K4SnO4) or have a spinel structure (e.g. Mg2SnO4) *''metastannates'' with a stoichiometry MIISnO3, MSnO3 which may contain polymeric anions or may be sometimes better described as mixed oxides These materials are semiconductors."Preparation, characterization and structure of metal stannates: a new family of photocatalysts for organic pollutants degradation." ''Handbook of Photocatalysts'' (2010), pp. 493–510. Nova Science Publishers, Inc. Nova Science Publishers is an academic publisher of books, encyclopedias, handbooks, e-books and journals, based in Hauppauge, New York. It was founded ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stannate
In chemistry the term stannate refers to compounds of tin (Sn). Stannic acid (Sn(OH)4), the formal precursor to stannates, does not exist and is actually a hydrate of SnO2. The term is also used in naming conventions as a suffix; for example the hexachlorostannate ion is . In materials science, two kinds of tin oxyanions are distinguished: *''orthostannates'' contain discrete units (e.g. K4SnO4) or have a spinel structure (e.g. Mg2SnO4) *''metastannates'' with a stoichiometry MIISnO3, MSnO3 which may contain polymeric anions or may be sometimes better described as mixed oxides These materials are semiconductors."Preparation, characterization and structure of metal stannates: a new family of photocatalysts for organic pollutants degradation." ''Handbook of Photocatalysts'' (2010), pp. 493–510. Nova Science Publishers, Inc. Nova Science Publishers is an academic publisher of books, encyclopedias, handbooks, e-books and journals, based in Hauppauge, New York. It was founded ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |