|
Silyl Enol Ethers
Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen end to an organosilicon group. They are important intermediates in organic synthesis. Synthesis Silyl enol ethers are generally prepared by reacting an enolizable carbonyl compound with a silyl electrophile and a base, or just reacting an enolate with a silyl electrophile.Clayden, J., Greeves, N., & Warren, S. (2012). Silyl enol ethers. In ''Organic chemistry'' (Second ed., pp. 466-467). Oxford University Press. Since silyl electrophiles are hard and silicon-oxygen bonds are very strong, the oxygen (of the carbonyl compound or enolate) acts as the nucleophile to form a Si-O single bond. The most commonly used silyl electrophile is trimethylsilyl chloride. To increase the rate of reaction, trimethylsilyl triflate may also be used in the place of trimethylsilyl chloride as a more electrophilic substrate. When using an unsymm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silyl Enol Ether
Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen end to an organosilicon group. They are important intermediates in organic synthesis. Synthesis Silyl enol ethers are generally prepared by reacting an enolizable carbonyl compound with a silyl electrophile and a base, or just reacting an enolate with a silyl electrophile.Clayden, J., Greeves, N., & Warren, S. (2012). Silyl enol ethers. In ''Organic chemistry'' (Second ed., pp. 466-467). Oxford University Press. Since silyl electrophiles are hard and silicon-oxygen bonds are very strong, the oxygen (of the carbonyl compound or enolate) acts as the nucleophile to form a Si-O single bond. The most commonly used silyl electrophile is trimethylsilyl chloride. To increase the rate of reaction, trimethylsilyl triflate may also be used in the place of trimethylsilyl chloride as a more electrophilic substrate. When using an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
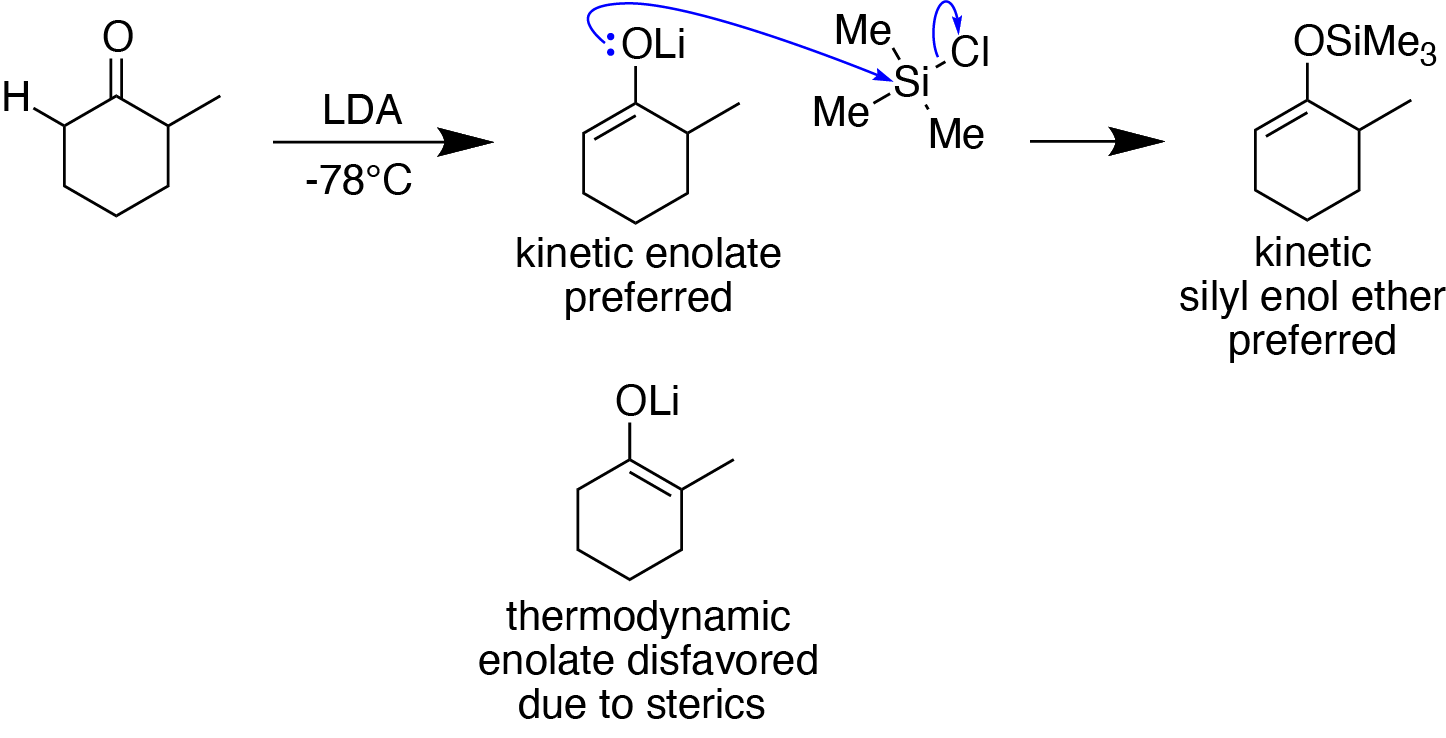
Example Synthesis Of A Kinetic Silyl Enol Ether
Example may refer to: * '' exempli gratia'' (e.g.), usually read out in English as "for example" * .example, reserved as a domain name that may not be installed as a top-level domain of the Internet ** example.com, example.net, example.org, example.edu, second-level domain names reserved for use in documentation as examples * HMS ''Example'' (P165), an Archer-class patrol and training vessel of the Royal Navy Arts * ''The Example'', a 1634 play by James Shirley * ''The Example'' (comics), a 2009 graphic novel by Tom Taylor and Colin Wilson * Example (musician), the British dance musician Elliot John Gleave (born 1982) * ''Example'' (album), a 1995 album by American rock band For Squirrels See also * * Exemplar (other), a prototype or model which others can use to understand a topic better * Exemplum, medieval collections of short stories to be told in sermons * Eixample The Eixample (; ) is a district of Barcelona between the old city (Ciutat Vella) and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mukaiyama Aldol Addition
The Mukaiyama aldol addition is an organic reaction and a type of aldol reaction between a silyl enol ether and an aldehyde or formate. The reaction was discovered by Teruaki Mukaiyama (1927–2018) in 1973. His choice of reactants allows for a crossed aldol reaction between an aldehyde and a ketone or a different aldehyde without self-condensation of the aldehyde. For this reason the reaction is used extensively in organic synthesis. General reaction scheme The Mukaiyama aldol addition is a Lewis acid mediated addition of enol silanes to carbonyl compounds. In this reaction compounds with various organic groups can be used (see educts). A basic version ( = H) without the presence of chiral catalysts is shown below. A racemic mix of enantiomers is built. If Z- or E-enol silanes are used in this reaction a mixture of four products occurs, yielding two racemates. Whether the ''anti''-diastereomer or the ''syn''-diastereomer is built depends largely on reaction conditions, sub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents. Nucleophilic alkylating agents Nucleophilic alkylating agents deliver the equivalent of an alkyl anion ( carbanion). The formal "alkyl anion" attacks an electrophile, forming a new cova ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Enolate Synthesis
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid element. Like all alkali metals, lithium is highly reactive and flammable, and must be stored in vacuum, inert atmosphere, or inert liquid such as purified kerosene or mineral oil. When cut, it exhibits a metallic luster, but moist air corrodes it quickly to a dull silvery gray, then black tarnish. It never occurs freely in nature, but only in (usually ionic) compounds, such as pegmatitic minerals, which were once the main source of lithium. Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. Lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride. The nucleus of the lithium atom verges on instability, since the two stable lithium isotopes found ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramethylsilane
Tetramethylsilane (abbreviated as TMS) is the organosilicon compound with the formula Si(CH3)4. It is the simplest tetraorganosilane. Like all silanes, the TMS framework is tetrahedral. TMS is a building block in organometallic chemistry but also finds use in diverse niche applications. Synthesis and reaction TMS is a by-product of the production of methyl chlorosilanes, SiCl''x''(CH3)4−''x'', via the direct process of reacting methyl chloride with silicon. The more useful products of this reaction are those for ''x'' = 1 (trimethylsilyl chloride), 2 (dimethyldichlorosilane), and 3 (methyltrichlorosilane). TMS undergoes deprotonation upon treatment with butyllithium to give (H3C)3SiCH2Li. The latter, trimethylsilylmethyl lithium, is a relatively common alkylating agent. In chemical vapor deposition, TMS is the precursor to silicon dioxide or silicon carbide, depending on the deposition conditions. Uses in NMR spectroscopy Tetramethylsilane is the accepted inter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic Substitution
In chemistry, a nucleophilic substitution is a class of chemical reaction A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and break ...s in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The molecule that contains the electrophile and the leaving functional group is called the Substrate (chemistry), substrate. The most general form of the reaction may be given as the following: :\text\mathbf + \ce + \text\mathbf The electron pair (:) from the nucleophile (Nuc) wiktionary:attack#Verb, attacks the substrate () and bonds with it. Simultaneously, the leaving group (LG) departs with an electron pair. The principal product in this case is . The nucleophile may be electricall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyllithium
Methyllithium is the simplest organolithium reagent with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used in solution with an ether as the solvent, is a reagent in organic synthesis as well as organometallic chemistry. Operations involving methyllithium require anhydrous conditions, because the compound is highly reactive toward water. Oxygen and carbon dioxide are also incompatible with MeLi. Methyllithium is usually not prepared, but purchased as a solution in various ethers. Synthesis In the direct synthesis, methyl bromide is treated with a suspension of lithium in diethyl ether. :2 Li + MeBr → LiMe + LiBr The lithium bromide forms a complex with the methyllithium. Most commercially available methyllithium consists of this complex. "Halide-free" methyllithium is prepared from methyl chloride. Lithium chloride precipitates from the diet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountered (e.g., ethylene dication ). Until the early 1970s, all carbocations were called ''carbonium ions''. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon: three in the carbenium ions and five in the carbonium ions. This nomenclature was proposed by G. A. Olah. Carbonium ions, as originally defined by Olah, are characterized by a three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called ' non-classical carbocations', which are carbocations that contain bridging C–C or C–H σ-bonds. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Acid Catalysis
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early (titanium, zirconium) and late (iron, copper, zinc) d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen (both sp2 or sp3), nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles. Many classical reactions involving carbon–carbon or carbon–heteroatom bond formation can be catalyzed by Lewis acids. Examples include the Friedel-Crafts reaction, the aldol reaction, and various pericyclic processes that proceed slowly at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres. Structure and bonding Aldehydes feature a carbon center that is connected by a double bond to oxygen and a single bond to hydrogen and single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2-Orbital hybridisation, hybridized. The aldehyde group is somewhat polar molecule, polar. The C=O bond length is about 120-122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enamine
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates. : The word "enamine" is derived from the affix ''en''-, used as the suffix of alkene, and the root ''amine''. This can be compared with enol, which is a functional group containing both alkene (''en''-) and alcohol (-''ol''). Enamines are considered to be nitrogen analogs of enols. If one of the nitrogen substituents is a hydrogen atom, H, it is the tautomeric form of an imine. This usually will rearrange to the imine; however there are several exceptions (such as aniline). The enamine-imine tautomerism may be considered analogous to the keto-enol tautomerism. In both cases, a hydrogen atom switches its location between the heteroatom (oxygen or nitrogen) and the second carbon atom. Enamines are both good nucleophiles and good bases. Their behavior as carbon-based nucleophiles is explained with reference to the following resona ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |