|
Silica Aerogel
Aerogels are a class of synthetic porous ultralight material derived from a gel, in which the liquid component for the gel has been replaced with a gas, without significant collapse of the gel structure. The result is a solid with extremely low density and extremely low thermal conductivity. Aerogels can be made from a variety of chemical compounds. Silica aerogels feel like fragile expanded polystyrene to the touch, while some polymer-based aerogels feel like rigid foams. The first documented example of an aerogel was created by Samuel Stephens Kistler in 1931, as a result of a bet with Charles Learned over who could replace the liquid in "jellies" with gas without causing shrinkage. Aerogels are produced by extracting the liquid component of a gel through supercritical drying or freeze-drying. This allows the liquid to be slowly dried off without causing the solid matrix in the gel to collapse from capillary action, as would happen with conventional evaporation. The first aer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aerogel Hand
Aerogels are a class of synthetic porous ultralight material derived from a gel, in which the liquid component for the gel has been replaced with a gas, without significant collapse of the gel structure. The result is a solid with extremely low density and extremely low thermal conductivity. Aerogels can be made from a variety of chemical compounds. Silica aerogels feel like fragile expanded polystyrene to the touch, while some polymer-based aerogels feel like rigid foams. The first documented example of an aerogel was created by Samuel Stephens Kistler in 1931, as a result of a bet with Charles Learned over who could replace the liquid in "jellies" with gas without causing shrinkage. Aerogels are produced by extracting the liquid component of a gel through supercritical drying or freeze-drying. This allows the liquid to be slowly dried off without causing the solid matrix in the gel to collapse from capillary action, as would happen with conventional evaporation. The fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silica Gel
Silica gel is an amorphous and porous form of silicon dioxide (silica), consisting of an irregular tridimensional framework of alternating silicon and oxygen atoms with nanometer-scale voids and pores. The voids may contain water or some other liquids, or may be filled by gas or vacuum. In the last case, the material is properly called silica xerogel. Silica xerogel with an average pore size of 2.4 nanometers has a strong affinity for water molecules and is widely used as a desiccant. It is hard and translucent, but considerably softer than massive silica glass or quartz; and remains hard when saturated with water. Silica xerogel is usually commercialized as coarse granules or beads, a few millimeters in diameter. Some grains may contain small amounts of indicator substance that changes color when they have absorbed some water. Small paper envelopes containing silica xerogel pellets, usually with a "do not eat" warning, are often included in dry food packages to absorb any ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Transfer
Heat transfer is a discipline of thermal engineering that concerns the generation, use, conversion, and exchange of thermal energy (heat) between physical systems. Heat transfer is classified into various mechanisms, such as thermal conduction, thermal convection, thermal radiation, and transfer of energy by phase changes. Engineers also consider the transfer of mass of differing chemical species (mass transfer in the form of advection), either cold or hot, to achieve heat transfer. While these mechanisms have distinct characteristics, they often occur simultaneously in the same system. Heat conduction, also called diffusion, is the direct microscopic exchanges of kinetic energy of particles (such as molecules) or quasiparticles (such as lattice waves) through the boundary between two systems. When an object is at a different temperature from another body or its surroundings, heat flows so that the body and the surroundings reach the same temperature, at which point they are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Insulation
Thermal insulation is the reduction of heat transfer (i.e., the transfer of thermal energy between objects of differing temperature) between objects in thermal contact or in range of radiative influence. Thermal insulation can be achieved with specially engineered methods or processes, as well as with suitable object shapes and materials. Heat flow is an inevitable consequence of contact between objects of different temperature. Thermal insulation provides a region of insulation in which thermal conduction is reduced, creating a thermal break or thermal barrier, or thermal radiation is reflected rather than absorbed by the lower-temperature body. The insulating capability of a material is measured as the inverse of thermal conductivity, thermal conductivity (k). Low thermal conductivity is equivalent to high insulating capability (R-value (insulation), resistance value). In thermal engineering, other important properties of insulating materials are product density, density (ρ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fractal
In mathematics, a fractal is a geometric shape containing detailed structure at arbitrarily small scales, usually having a fractal dimension strictly exceeding the topological dimension. Many fractals appear similar at various scales, as illustrated in successive magnifications of the Mandelbrot set. This exhibition of similar patterns at increasingly smaller scales is called self-similarity, also known as expanding symmetry or unfolding symmetry; if this replication is exactly the same at every scale, as in the Menger sponge, the shape is called affine self-similar. Fractal geometry lies within the mathematical branch of measure theory. One way that fractals are different from finite geometric figures is how they scale. Doubling the edge lengths of a filled polygon multiplies its area by four, which is two (the ratio of the new to the old side length) raised to the power of two (the conventional dimension of the filled polygon). Likewise, if the radius of a filled s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
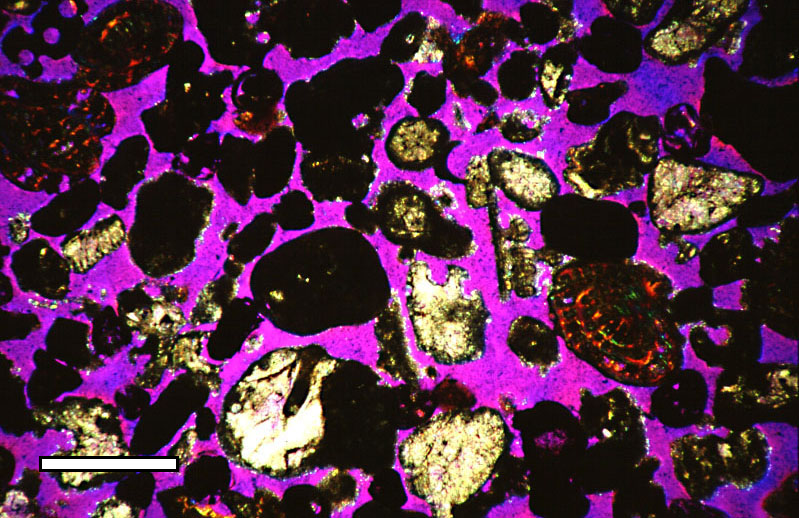
Porosity
Porosity or void fraction is a measure of the void (i.e. "empty") spaces in a material, and is a fraction of the volume of voids over the total volume, between 0 and 1, or as a percentage between 0% and 100%. Strictly speaking, some tests measure the "accessible void", the total amount of void space accessible from the surface (cf. closed-cell foam). There are many ways to test porosity in a substance or part, such as industrial CT scanning. The term porosity is used in multiple fields including pharmaceutics, ceramics, metallurgy, materials, manufacturing, petrophysics, hydrology, earth sciences, soil mechanics, and engineering. Void fraction in two-phase flow In gas-liquid two-phase flow, the void fraction is defined as the fraction of the flow-channel volume that is occupied by the gas phase or, alternatively, as the fraction of the cross-sectional area of the channel that is occupied by the gas phase. Void fraction usually varies from location to location in the fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanometre
330px, Different lengths as in respect to the Molecule">molecular scale. The nanometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: nm) or nanometer ( American spelling) is a unit of length in the International System of Units (SI), equal to one billionth ( short scale) of a metre () and to 1000 picometres. One nanometre can be expressed in scientific notation as , and as metres. History The nanometre was formerly known as the millimicrometre – or, more commonly, the millimicron for short – since it is of a micron (micrometre), and was often denoted by the symbol mμ or (more rarely and confusingly, since it logically should refer to a ''millionth'' of a micron) as μμ. Etymology The name combines the SI prefix '' nano-'' (from the Ancient Greek , ', "dwarf") with the parent unit name ''metre'' (from Greek , ', "unit of measurement"). Usage Nanotechnologies are based on phenomena typically occurr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spherical
A sphere () is a geometrical object that is a three-dimensional analogue to a two-dimensional circle. A sphere is the set of points that are all at the same distance from a given point in three-dimensional space.. That given point is the centre of the sphere, and is the sphere's radius. The earliest known mentions of spheres appear in the work of the ancient Greek mathematicians. The sphere is a fundamental object in many fields of mathematics. Spheres and nearly-spherical shapes also appear in nature and industry. Bubbles such as soap bubbles take a spherical shape in equilibrium. The Earth is often approximated as a sphere in geography, and the celestial sphere is an important concept in astronomy. Manufactured items including pressure vessels and most curved mirrors and lenses are based on spheres. Spheres roll smoothly in any direction, so most balls used in sports and toys are spherical, as are ball bearings. Basic terminology As mentioned earlier is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dendrite (metal)
A dendrite in metallurgy is a characteristic tree-like structure of crystals growing as molten metal solidifies, the shape produced by faster growth along energetically favourable crystallographic directions. This dendritic growth has large consequences in regard to material properties. Dendrites form in unary (one-component) systems as well as multi-component systems. The requirement is that the liquid (the molten material) be undercooled, aka supercooled, below the freezing point of the solid. Initially, a spherical solid nucleus grows in the undercooled melt. As the sphere grows, the spherical morphology becomes unstable and its shape becomes perturbed. The solid shape begins to express the preferred growth directions of the crystal. This growth direction may be due to anisotropy in the surface energy of the solid–liquid interface, or to the ease of attachment of atoms to the interface on different crystallographic planes, or both (for an example of the latter, see hop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Friability
Friability ( ), the condition of being friable, describes the tendency of a solid substance to break into smaller pieces under duress or contact, especially by rubbing. The opposite of friable is indurate. Substances that are designated hazardous, such as asbestos or crystalline silica, are often said to be friable if small particles are easily dislodged and become airborne, and hence respirable (able to enter human lungs), thereby posing a health hazard. Tougher substances, such as concrete, may also be mechanically ground down and reduced to finely divided mineral dust. However, such substances are not generally considered friable because of the degree of difficulty involved in breaking the substance's chemical bonds through mechanical means. Some substances, such as polyurethane foams, show an increase in friability with exposure to ultraviolet radiation, as in sunlight. Friable is sometimes used metaphorically to describe "brittle" personalities who can be "rubbed" by seemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of Earth's crust. Three isotopes occur naturally, C and C being stable, while C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the few elements known since antiquity. Carbon is the 15th most abundant element in the Earth's crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual ability to form polymers at the temperatures commonly encountered on Earth, enables this element to serve as a common element of Carbon-based life, all known life. It is the second most abundant element in the human body by mass (about 18.5%) after oxygen. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |