|
Salicide
The term salicide refers to a technology used in the microelectronics industry used to form electrical contacts between the semiconductor device and the supporting interconnect structure. The salicide process involves the reaction of a metal thin film with silicon in the active regions of the device, ultimately forming a metal silicide contact through a series of annealing and/or etch processes. The term "salicide" is a compaction of the phrase self-aligned silicide. The description "self-aligned" suggests that the contact formation does not require photolithography patterning processes, as opposed to a non-aligned technology such as polycide. The term salicide is also used to refer to the metal silicide formed by the contact formation process, such as "titanium salicide", although this usage is inconsistent with accepted naming conventions in chemistry. Contact Formation The salicide process begins with deposition of a thin transition metal layer over fully formed and pat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polycide
Polycide is a silicide formed over polysilicon. Widely used in DRAMs. In a polycide MOSFET transistor process, the silicide is formed only over the polysilicon film as formation occurs prior to any polysilicon etch. Polycide processes contrast with salicide The term salicide refers to a technology used in the microelectronics industry used to form electrical contacts between the semiconductor device and the supporting interconnect structure. The salicide process involves the reaction of a metal th ... processes in which silicide is formed after the polysilicon etch. Thus, with a salicide process, silicide is formed over both the polysilicon gate and the exposed monocrystalline terminal regions of the transistor in a self-aligned fashion. {{Chemistry-stub Semiconductor device fabrication Silicon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Disilicide
Titanium disilicide ( Ti Si2) is an inorganic chemical compound of titanium and silicon. Preparation Titanium disilicide can be obtained from the reaction between titanium or titanium hydride with silicon. :Ti + 2 Si → TiSi2 It is also possible to prepare it aluminothermically by the ignition of aluminium powder, sulfur, silicon dioxide, and titanium dioxide or potassium hexafluorotitanate, K2TiF6, by electrolysis of a melt of potassium hexafluorotitanate and titanium dioxide, or by reaction of titanium with silicon tetrachloride. Another method is the reaction of titanium tetrachloride with silane, dichlorosilane or silicon. :TiCl4 + 2 SiH4 → TiSi2 + 4 HCl + 2 H2 :TiCl4 + 2 SiH2Cl2 + 2 H2 → TiSi2 + 8 HCl :TiCl4 + 3 Si → TiSi2 + SiCl4 Uses Titanium silicide is used in the semiconductor industry. It is typically grown by means of salicide The term salicide refers to a technology used in the microelectronics industry used to form electrical contacts between the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicide
A silicide is a type of chemical compound that combines silicon and a (usually) more electropositive element. Silicon is more electropositive than carbon. Silicides are structurally closer to borides than to carbides. Similar to borides and carbides, the composition of silicides cannot be easily specified as covalent molecules. The chemical bonds in silicides range from conductive metal-like structures to covalent or ionic. Silicides of all non-transition metals, with exception of beryllium, have been described. Overview Silicon atoms in silicides can have many possible organizations: *Isolated silicon atoms: electrically conductive (or semiconductive) CrSi, MnSi, FeSi, CoSi, , , , , and nonconductive , *Si2 pairs: , hafnium and thorium silicides *Si4 tetrahedra: KSi, RbSi, CsSi *Si''n'' chains: USi, , CaSi, SrSi, YSi *Planar hexagonal graphite-like Si layers: β-USi2, silicides of other lanthanoids and actinoids *Corrugated hexagonal Si layers: CaSi2 *Open three-dimensio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microelectronics
Microelectronics is a subfield of electronics. As the name suggests, microelectronics relates to the study and manufacture (or microfabrication) of very small electronic designs and components. Usually, but not always, this means micrometre-scale or smaller. These devices are typically made from semiconductor materials. Many components of normal electronic design are available in a microelectronic equivalent. These include transistors, capacitors, inductors, resistors, diodes and (naturally) insulators and conductors can all be found in microelectronic devices. Unique wiring techniques such as wire bonding are also often used in microelectronics because of the unusually small size of the components, leads and pads. This technique requires specialized equipment and is expensive. Digital integrated circuits (ICs) consist of billions of transistors, resistors, diodes, and capacitors. Analog circuits commonly contain resistors and capacitors as well. Inductors are used in som ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
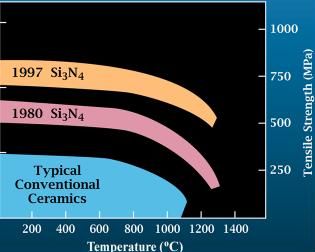
Silicon Nitride
Silicon nitride is a chemical compound of the elements silicon and nitrogen. is the most thermodynamically stable and commercially important of the silicon nitrides, and the term "silicon nitride" commonly refers to this specific composition. It is a white, high-melting-point solid that is relatively chemically inert, being attacked by dilute HF and hot . It is very hard (8.5 on the mohs scale). It has a high thermal stability with strong optical nonlinearities for all-optical applications. Production Silicon nitride is prepared by heating powdered silicon between 1300 °C and 1400 °C in a nitrogen atmosphere: :3 Si + 2 → The silicon sample weight increases progressively due to the chemical combination of silicon and nitrogen. Without an iron catalyst, the reaction is complete after several hours (~7), when no further weight increase due to nitrogen absorption (per gram of silicon) is detected. In addition to , several other silicon nitride phases (with chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Self-aligned Gate
In semiconductor electronics fabrication technology, a self-aligned gate is a transistor manufacturing approach whereby the gate electrode of a MOSFET (metal–oxide–semiconductor field-effect transistor) is used as a mask for the doping of the source and drain regions. This technique ensures that the gate is naturally and precisely aligned to the edges of the source and drain. The use of self-aligned gates in MOS transistors is one of the key innovations that led to the large increase in computing power in the 1970s. Self-aligned gates are still used in most modern integrated circuit processes. Introduction IC construction Integrated circuits (ICs, or "chips") are produced in a multi-step process that builds up multiple layers on the surface of a disk of silicon known as a "wafer". Each layer is patterned by coating the wafer in photoresist and then exposing it to ultraviolet light being shone through a stencil-like "mask". Depending on the process, the photoresist that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Short Circuit
A short circuit (sometimes abbreviated to short or s/c) is an electrical circuit that allows a current to travel along an unintended path with no or very low electrical impedance. This results in an excessive current flowing through the circuit. The opposite of a short circuit is an "open circuit", which is an infinite resistance between two nodes. Definition A short circuit is an abnormal connection between two nodes of an electric circuit intended to be at different voltages. This results in an electric current limited only by the Thévenin equivalent resistance of the rest of the network which can cause circuit damage, overheating, fire or explosion. Although usually the result of a fault, there are cases where short circuits are caused intentionally, for example, for the purpose of voltage-sensing crowbar circuit protectors. In circuit analysis, a ''short circuit'' is defined as a connection between two nodes that forces them to be at the same voltage. In an 'ideal' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metastability
In chemistry and physics, metastability denotes an intermediate Energy level, energetic state within a dynamical system other than the system's ground state, state of least energy. A ball resting in a hollow on a slope is a simple example of metastability. If the ball is only slightly pushed, it will settle back into its hollow, but a stronger push may start the ball rolling down the slope. Bowling pins show similar metastability by either merely wobbling for a moment or tipping over completely. A common example of metastability in science is isomerisation. Higher energy isomers are long lived because they are prevented from rearranging to their preferred ground state by (possibly large) barriers in the potential energy. During a metastable state of finite lifetime, all state-describing parameters reach and hold stationary values. In isolation: *the state of least energy is the only one the system will inhabit for an indefinite length of time, until more external energy is added ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Stability
In chemistry, chemical stability is the thermodynamic stability of a chemical system. Thermodynamic stability occurs when a system is in its lowest energy state, or in chemical equilibrium with its environment. This may be a dynamic equilibrium in which individual atoms or molecules change form, but their overall number in a particular form is conserved. This type of chemical thermodynamic equilibrium will persist indefinitely unless the system is changed. Chemical systems might undergo changes in the phase of matter or a set of chemical reactions. State A is said to be more thermodynamically stable than state B if the Gibbs free energy of the change from A to B is positive. Versus reactivity Thermodynamic stability applies to a particular system. The reactivity of a chemical substance is a description of how it might react across a variety of potential chemical systems and, for a given system, how fast such a reaction could proceed. Chemical substances or states can pers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isolated as a metal in 1783. Its important ores include scheelite and wolframite, the latter lending the element its alternate name. The free element is remarkable for its robustness, especially the fact that it has the highest melting point of all known elements barring carbon (which sublimes at normal pressure), melting at . It also has the highest boiling point, at . Its density is , comparable with that of uranium and gold, and much higher (about 1.7 times) than that of lead. Polycrystalline tungsten is an intrinsically brittle and hard material (under standard conditions, when uncombined), making it difficult to work. However, pure single-crystalline tungsten is more ductile and can be cut with a hard-steel hacksaw. Tungsten occurs in many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver". Platinum is a member of the platinum group of elements and group 10 of the periodic table of elements. It has six naturally occurring isotopes. It is one of the rarer elements in Earth's crust, with an average abundance of approximately 5 μg/kg. It occurs in some nickel and copper ores along with some native deposits, mostly in South Africa, which accounts for ~80% of the world production. Because of its scarcity in Earth's crust, only a few hundred tonnes are produced annually, and given its important uses, it is highly valuable and is a major precious metal commodity. Platinum is one of the least reactive metals. It has remarkable resistance to corrosion, even at high temperatures, and is therefore considered a noble metal. Consequent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to react with air under standard conditions because a passivation layer of nickel oxide forms on the surface that prevents further corrosion. Even so, pure native nickel is found in Earth's crust only in tiny amounts, usually in ultramafic rocks, and in the interiors of larger nickel–iron meteorites that were not exposed to oxygen when outside Earth's atmosphere. Meteoric nickel is found in combination with iron, a reflection of the origin of those elements as major end products of supernova nucleosynthesis. An iron–nickel mixture is thought to compose Earth's outer and inner cores. Use of nickel (as natural meteoric nickel–iron alloy) has been traced as far back as 3500 BCE. Nickel was first isolated and classified as an e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |