|
Run-in Period
Run-in period is a period between the recruitment and randomization phases of a clinical trial, when all participants receive the same treatment, which may be active treatment, a placebo or no treatment at all. The clinical data from this stage of a trial are only occasionally of value but can serve a valuable role in screening out ineligible or non-compliant participants, in ensuring that participants are in a stable condition, and in providing baseline observations. A run-in period is sometimes called a ''washout period'' if treatments that participants were using before entering the clinical trial are discontinued. See also *Data management *Randomized controlled trial * Regulatory requirement *Safety monitoring *Serious adverse event *Standard operating procedures *Standard treatment *Study population Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral intervent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phases Of Clinical Research
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays. Summary Clinical trials testing potential medical products are commonly classified into four phases. The drug development process will normally proceed through all four phases over many years. If the drug successfully passes through Phases I, II, and III, it will usually be approved by the national regulatory authority for use in the general population. Phase IV trials are 'post-marketing' or 'surveillance' studies conducted to monitor safety over sever ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
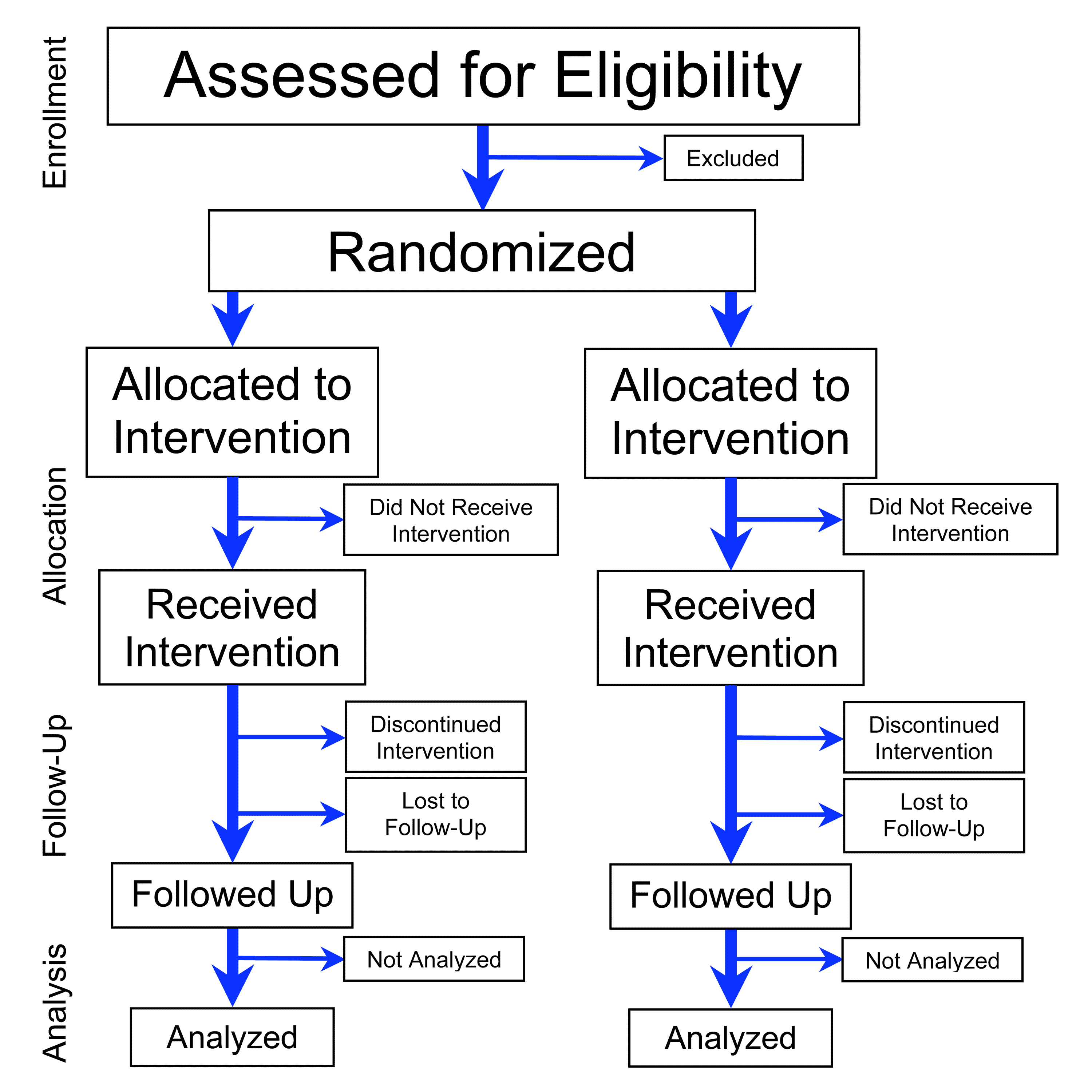
Clinical Trial
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small pilot studies, and subsequently conduct progressively larger scale comparative studies. Clinical trials can vary i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Placebo
A placebo ( ) is a substance or treatment which is designed to have no therapeutic value. Common placebos include inert tablets (like sugar pills), inert injections (like Saline (medicine), saline), sham surgery, and other procedures. In general, placebos can affect how patients perceive their condition and encourage the body's chemical processes for relieving pain and a few other symptoms, but have no impact on the disease itself. Improvements that patients experience after being treated with a placebo can also be due to unrelated factors, such as regression to the mean (a statistical effect where an unusually high or low measurement is likely to be followed by a less extreme one). The use of placebos in clinical medicine raises ethical concerns, especially if they are disguised as an active treatment, as this introduces dishonesty into the doctor–patient relationship and bypasses informed consent. While it was once assumed that this deception was necessary for placebos to have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Data
Clinical may refer to: Healthcare * Of or about a clinic, a healthcare facility * Of or about the practice of medicine Other uses * ''Clinical'' (film), a 2017 American horror thriller See also * * * Clinical chemistry, the analysis of bodily fluids for diagnostic and therapeutic purposes * Clinical death, the cessation of blood circulation and breathing * Clinical formulation, a theoretically-based explanation of information obtained from clinical assessment * Clinical governance, a systematic approach to maintaining and improving the quality of patient care * Clinical linguistics, linguistics applied to speech-language pathology * Clinical psychology, the understanding, preventing, and relieving psychologically-based distress or dysfunction * Clinical research, to determine the safety and effectiveness of medications etc. * Clinical significance, the practical importance of a treatment effect * Clinical trial, experiments or observations done in clinical research * Clinical wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Screening (medicine)
Screening, in medicine, is a strategy used to look for as-yet-unrecognised conditions or risk markers. This testing can be applied to individuals or to a whole population. The people tested may not exhibit any signs or symptoms of a disease, or they might exhibit only one or two symptoms, which by themselves do not indicate a definitive diagnosis. Screening interventions are designed to identify conditions which could at some future point turn into disease, thus enabling earlier intervention and management in the hope to reduce mortality and suffering from a disease. Although screening may lead to an earlier diagnosis, not all screening tests have been shown to benefit the person being screened; overdiagnosis, misdiagnosis, and creating a false sense of security are some potential adverse effects of screening. Additionally, some screening tests can be inappropriately overused. For these reasons, a test used in a screening program, especially for a disease with low incidence, must ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Data Management
Data management comprises all disciplines related to handling data as a valuable resource. Concept The concept of data management arose in the 1980s as technology moved from sequential processing (first punched cards, then magnetic tape) to random access storage. Since it was now possible to store a discrete fact and quickly access it using random access disk technology, those suggesting that data management was more important than business process management used arguments such as "a customer's home address is stored in 75 (or some other large number) places in our computer systems." However, during this period, random access processing was not competitively fast, so those suggesting "process management" was more important than "data management" used batch processing time as their primary argument. As application software evolved into real-time, interactive usage, it became obvious that both management processes were important. If the data was not well defined, the data wo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Randomized Controlled Trial
A randomized controlled trial (or randomized control trial; RCT) is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures or other medical treatments. Participants who enroll in RCTs differ from one another in known and unknown ways that can influence study outcomes, and yet cannot be directly controlled. By Random assignment, randomly allocating participants among compared treatments, an RCT enables ''statistical control'' over these influences. Provided it is designed well, conducted properly, and enrolls enough participants, an RCT may achieve sufficient control over these confounding factors to deliver a useful comparison of the treatments studied. Definition and examples An RCT in clinical research typically compares a proposed new treatment against an existing Standard of care#Medical standard of care, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Regulatory Requirement
Regulation is the management of complex systems according to a set of rules and trends. In systems theory, these types of rules exist in various fields of biology and society, but the term has slightly different meanings according to context. For example: * in biology, gene regulation and metabolic regulation allow living organisms to adapt to their environment and maintain homeostasis; * in government, typically regulation means stipulations of the delegated legislation which is drafted by subject-matter experts to enforce primary legislation; * in business, industry self-regulation occurs through self-regulatory organizations and trade associations which allow industries to set and enforce rules with less government involvement; and, * in psychology, self-regulation theory is the study of how individuals regulate their thoughts and behaviors to reach goals. Social Regulation in the social, political, psychological, and economic domains can take many forms: legal restrictions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Safety Monitoring
Clinical monitoring is the oversight and administrative efforts that monitor a participant's health and efficacy of the treatment during a clinical trial. Both independent and government-run grant-funding agencies, such as the National Institutes of Health, National Institutes of Health (NIH) and the World Health Organization, World Health Organization (WHO), require data and safety monitoring protocols for Phase I and II clinical trials conforming to their standards. Safety monitoring Safety monitoring of a clinical trial is conducted by an independent physician with relevant expertise. This is accomplished by review of adverse event, immediately after they occur, with timely follow-up through resolution. Responsibility for data and safety monitoring depends on the phase of the study and may be conducted by sponsor or Contract research organization (CRO) staff or contractor, and/or by the Principal clinical investigator/project manager conducting the study. Regardless of the metho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serious Adverse Event
A serious adverse event (SAE) in human drug trials is defined as any untoward medical occurrence that at any dose #Results in death #Is life-threatening #Requires inpatient hospitalization or causes prolongation of existing hospitalization #Results in persistent or significant disability/incapacity #May have caused a congenital anomaly/birth defect #Requires intervention to prevent permanent impairment or damage The term "life-threatening" in the definition of "serious" refers to an event in which the patient was at risk of death at the time of the event; it does not refer to an event which hypothetically might have caused death if it were more severe. Adverse events are further defined as “Any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have to have a causal relationship with this treatment.” Research Investigators in human clinical trials are obligated to report these events in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Standard Operating Procedures
A standard operating procedure (SOP) is a set of step-by-step instructions compiled by an organization to help workers carry out routine operations. SOPs aim to achieve efficiency, quality output, and uniformity of performance, while reducing miscommunication and failure to comply with industry regulations. Some military services (e.g., in the U.S. and the UK) use the term standing (rather than ''standard'') operating procedure, since a military SOP refers to a unit's unique procedures, which are not necessarily standard to another unit. The word "standard" could suggest that only one (standard) procedure is to be used across all units. The term is sometimes used facetiously to refer to practices that are unconstructive, yet the norm. In the Philippines, for instance, "SOP" is the term for pervasive corruption within the government and its institutions. Clinical research and practice In clinical research, the '' International Council for Harmonisation'' (ICH) defines SOPs as "de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Standard Treatment
The standard treatment, also known as the standard of care, is the medical treatment that is normally provided to people with a given condition. In many scientific studies, the control group receives the standard treatment rather than a placebo while a treatment group receives the experimental treatment. After the clinical trial, researchers compare the outcomes of the two groups to see if the experimental treatment is better than, as good as or not as beneficial as the standard treatment. Active (positive) concurrent control In an active control or positive control trial, subjects are randomly assigned to the test treatment or to an active control treatment. Such clinical trials are usually double-blind, but this is not always possible; many oncology trials, for example, are considered difficult or impossible to blind because of different regimens, different routes of administration, and different toxicities. Active control trials can have two distinct objectives with respect t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)

.jpg)