|
Receptor Theory
Receptor theory is the application of receptor models to explain drug behavior. Pharmacological receptor models preceded accurate knowledge of receptors by many years. John Newport Langley and Paul Ehrlich introduced the concept of a receptor that would mediate drug action at the beginning of the 20th century. Alfred Joseph Clark was the first to quantify drug-induced biological responses (specifically, f-mediated receptor activation). So far, nearly all of the quantitative theoretical modelling of receptor function has centred on ligand-gated ion channels and GPCRs. History The receptor concept In 1901, Langley challenged the dominant hypothesis that drugs act at nerve endings by demonstrating that nicotine acted at sympathetic ganglia even after the degeneration of the severed preganglionic nerve endings. In 1905 he introduced the concept of a receptive substance on the surface of skeletal muscle that mediated the action of a drug. Langley postulated that these receptive substan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor (biochemistry)
In biochemistry and pharmacology, receptors are chemical structures, composed of protein, that receive and transduce signals that may be integrated into biological systems. These signals are typically chemical messengers which bind to a receptor and cause some form of cellular/tissue response, e.g. a change in the electrical activity of a cell. There are three main ways the action of the receptor can be classified: relay of signal, amplification, or integration. Relaying sends the signal onward, amplification increases the effect of a single ligand, and integration allows the signal to be incorporated into another biochemical pathway. Receptor proteins can be classified by their location. Transmembrane receptors include ligand-gated ion channels, G protein-coupled receptors, and enzyme-linked hormone receptors. Intracellular receptors are those found inside the cell, and include cytoplasmic receptors and nuclear receptors. A molecule that binds to a receptor is called a ligan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schild Regression
In pharmacology, Schild regression analysis, named for Heinz Otto Schild, is a tool for studying the effects of agonists and antagonists on the response caused by the receptor or on ligand-receptor binding. Dose-response curves can be constructed to describe response or ligand-receptor complex formation as a function of the ligand concentration. Antagonists make it harder to form these complexes by inhibiting interactions of the ligand with its receptor. This is seen as a change in the dose response curve: typically a rightward shift or a lowered maximum. A reversible competitive antagonist should cause a rightward shift in the dose response curve, such that the new curve is parallel to the old one and the maximum is unchanged. This is because reversible competitive antagonists are surmountable antagonists. The magnitude of the rightward shift can be quantified with the dose ratio, r. The dose ratio r is the ratio of the dose of agonist required for half maximal response wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and metabolism. Over the last decades of the 20th century, biochemistry has become successful at explaining living processes through these three disciplines. Almost all areas of the life sciences are being uncovered and developed through biochemical methodology and research. Voet (2005), p. 3. Biochemistry focuses on understanding the chemical basis which allows biological molecules to give rise to the processes that occur within living cells and between cells, Karp (2009), p. 2. in turn relating greatly to the understanding of tissues and organs, as well as organism structure and function.Miller (2012). p. 62. Biochemistry is closely related to molecular biology, which is the study of the molecular mechanisms of biological phenomena.Ast ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dissociation Constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (K_D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into its component ions. The dissociation constant is the inverse of the association constant. In the special case of salts, the dissociation constant can also be called an ionization constant. For a general reaction: : A_\mathit B_\mathit \mathit A + \mathit B in which a complex \ce_x \ce_y breaks down into ''x'' A subunits and ''y'' B subunits, the dissociation constant is defined as : K_D = \frac where and ''x'' B''y''are the equilibrium concentrations of A, B, and the complex A''x'' B''y'', respectively. One reason for the popularity of the dissociation constant in biochemistry and pharmacology is that in the frequently enc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding Sites
In biochemistry and molecular biology, a binding site is a region on a macromolecule such as a protein that binds to another molecule with specificity. The binding partner of the macromolecule is often referred to as a ligand. Ligands may include other proteins (resulting in a protein-protein interaction), enzyme substrates, second messengers, hormones, or allosteric modulators. The binding event is often, but not always, accompanied by a conformational change that alters the protein's function. Binding to protein binding sites is most often reversible (transient and non-covalent), but can also be covalent reversible or irreversible. Function Binding of a ligand to a binding site on protein often triggers a change in conformation in the protein and results in altered cellular function. Hence binding site on protein are critical parts of signal transduction pathways. Types of ligands include neurotransmitters, toxins, neuropeptides, and steroid hormones. Binding sites incur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor (biochemistry)
In biochemistry and pharmacology, receptors are chemical structures, composed of protein, that receive and transduce signals that may be integrated into biological systems. These signals are typically chemical messengers which bind to a receptor and cause some form of cellular/tissue response, e.g. a change in the electrical activity of a cell. There are three main ways the action of the receptor can be classified: relay of signal, amplification, or integration. Relaying sends the signal onward, amplification increases the effect of a single ligand, and integration allows the signal to be incorporated into another biochemical pathway. Receptor proteins can be classified by their location. Transmembrane receptors include ligand-gated ion channels, G protein-coupled receptors, and enzyme-linked hormone receptors. Intracellular receptors are those found inside the cell, and include cytoplasmic receptors and nuclear receptors. A molecule that binds to a receptor is called a ligan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
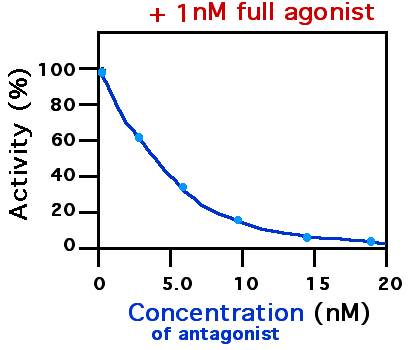
Receptor Antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves " '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, beta blocker [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
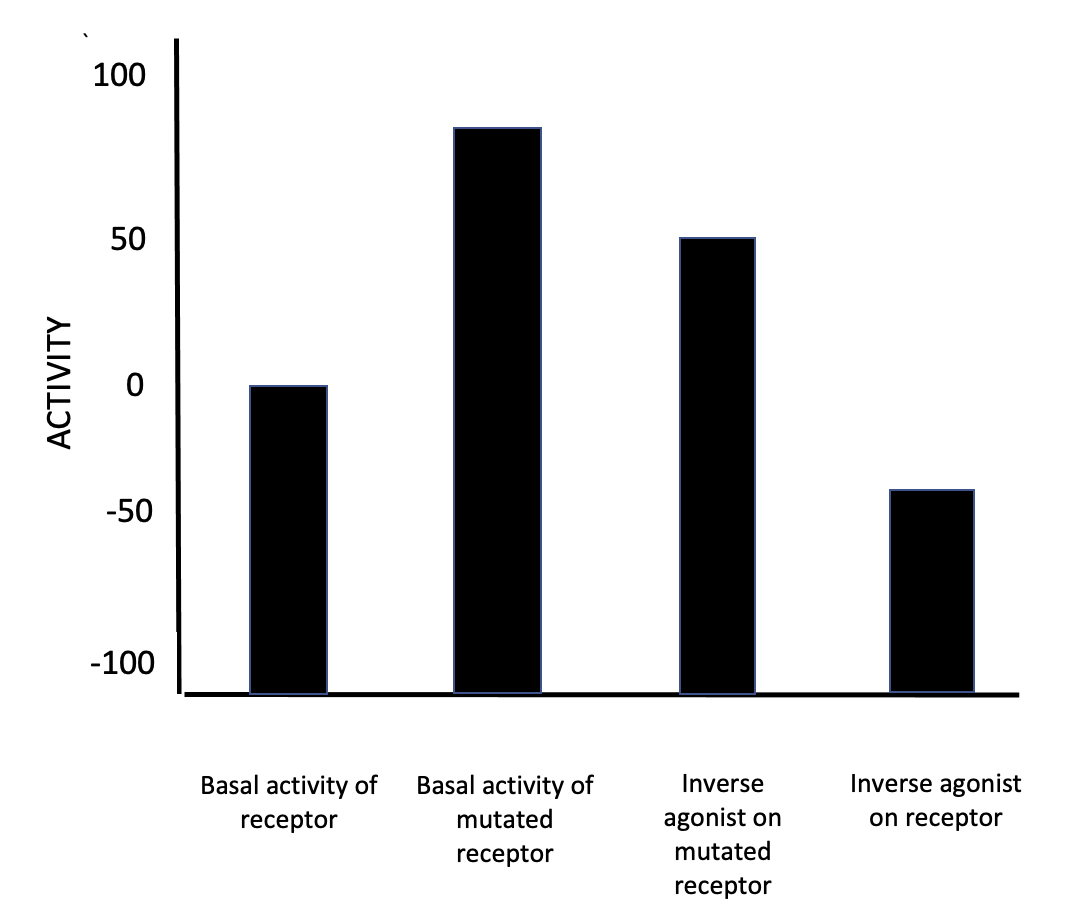
Inverse Agonists
In pharmacology, an inverse agonist is a drug that binds to the same receptor as an agonist but induces a pharmacological response opposite to that of the agonist. A neutral antagonist has no activity in the absence of an agonist or inverse agonist but can block the activity of either. Inverse agonists have opposite actions to those of agonists but the effects of both of these can be blocked by antagonists. A prerequisite for an inverse agonist response is that the receptor must have a constitutive (also known as intrinsic or basal) level of activity in the absence of any ligand. An agonist increases the activity of a receptor above its basal level, whereas an inverse agonist decreases the activity below the basal level. The efficacy of a full agonist is by definition 100%, a neutral antagonist has 0% efficacy, and an inverse agonist has < 0% (i.e., negative) efficacy. Examples Receptors for which inverse agonists have been identified include the[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agonists
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology From the Greek αγωνιστής (agōnistēs), contestant; champion; rival < αγων (agōn), contest, combat; exertion, struggle < αγω (agō), I lead, lead towards, conduct; drive Types of agonists Receptors can be activated by either agonists (such as |
Protein Structure
Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, the monomers of the polymer. A single amino acid monomer may also be called a ''residue'' indicating a repeating unit of a polymer. Proteins form by amino acids undergoing condensation reactions, in which the amino acids lose one water molecule per reaction in order to attach to one another with a peptide bond. By convention, a chain under 30 amino acids is often identified as a peptide, rather than a protein. To be able to perform their biological function, proteins fold into one or more specific spatial conformations driven by a number of non-covalent interactions such as hydrogen bonding, ionic interactions, Van der Waals forces, and hydrophobic packing. To understand the functions of proteins at a molecular level, it is often necessary to determine their three-dimensional structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology From the Greek αγωνιστής (agōnistēs), contestant; champion; rival < αγων (agōn), contest, combat; exertion, struggle < αγω (agō), I lead, lead towards, conduct; drive Types of agonists can be activated by either endogenous agonists (such as[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Everhardus Jacobus Ariëns
Everhardus Jacobus Ariëns (29 January 1918 – 3 March 2002) was a Dutch pharmacologist and professor at the Catholic University of Nijmegen (now Radboud University Nijmegen). He made important contributions to the function of receptors and the mathematical description of ligand–receptor interactions (receptor theory). Moreover, Everhardus Ariëns was the initiator for the collection of stereochemistry in drug development and spearheading the development of enantiopure drugs. Early life Everhardus Ariëns grew up as the sixth of ten children in Wijk bij Duurstede. After a temporary boarding school experience, in 1935 he was admitted to Wageningen, the general university. Then he took a degree in chemistry at the University of Utrecht in which he completed in 1942, although his preference was actually the biology. Another study was interrupted by the Second World War. After his refusal to sign a declaration of loyalty to the German Reich and an escape from the Germany o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |