|
Rule Of Mixtures
In materials science, a general rule of mixtures is a weighted mean used to predict various properties of a composite material . It provides a theoretical upper- and lower-bound on properties such as the elastic modulus, mass density, ultimate tensile strength, thermal conductivity, and electrical conductivity. In general there are two models, one for axial loading (Voigt model), and one for transverse loading (Reuss model). In general, for some material property E (often the elastic modulus), the rule of mixtures states that the overall property in the direction parallel to the fibers may be as high as : E_c = fE_f + \left(1-f\right)E_m where * f = \frac is the volume fraction of the fibers * E_f is the material property of the fibers * E_m is the material property of the matrix It is a common mistake to believe that this is the upper-bound modulus for Young's modulus. The real upper-bound Young's modulus is larger than E_c given by this formula. Even if both constituents are is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Composite Elastic Modulus
Composite or compositing may refer to: Materials * Composite material, a material that is made from several different substances ** Metal matrix composite, composed of metal and other parts ** Cermet, a composite of ceramic and metallic materials ** Dental composite, a substance used to fill cavities in teeth ** Composite armor, a type of tank armor * Alloy, a mixture of a metal and another element * Mixture, the combination of several different substances without chemical reaction Mathematics * Composite number, a positive integer that has at least one factor other than one or itself Science * Composite particle, a particle which is made up of smaller particles * ''Compositae'' or "composite family" of flowering plants * Composite volcano, a layered conical volcano * Compositing, another name for superposed epoch analysis, a statistical method used to analyze time series involving multiple events Technology * Compositing, combining of visual elements from separate sources into ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volume Fraction
In chemistry and fluid mechanics, the volume fraction φ''i'' is defined as the volume of a constituent ''V''''i'' divided by the volume of all constituents of the mixture ''V'' prior to mixing: :\phi_i = \frac Being dimensionless, its unit is 1; it is expressed as a number, e.g., 0.18. It is the same concept as volume percent (vol%) except that the latter is expressed with a denominator of 100, e.g., 18%. The volume fraction coincides with the volume concentration in ideal solutions where the volumes of the constituents are additive (the volume of the solution is equal to the sum of the volumes of its ingredients). The sum of all volume fractions of a mixture is equal to 1: :\sum_^ V_i = V ; \qquad \sum_^ \phi_i = 1 The volume fraction (percentage by volume, vol%) is one way of expressing the composition of a mixture with a dimensionless quantity; mass fraction (percentage by weight, wt%) and mole fraction (percentage by moles, mol%) are others. Volume concentration and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vegard's Law
In crystallography, materials science and metallurgy, Vegard's law is an empirical finding (heuristic approach) resembling the rule of mixtures. In 1921, Lars Vegard discovered that the lattice parameter of a solid solution of two constituents is approximately a weighted mean of the two constituents' lattice parameters at the same temperature: :a_ = (1-x)\ a_\mathrm + x\ a_\mathrm e.g., in the case of a mixed oxide of uranium and plutonium as used in the fabrication of MOX nuclear fuel: :a_\mathrm = 0.93\ a_\mathrm + 0.07\ a_\mathrm Vegard's law assumes that both components A and B in their pure form (i.e. before mixing) have the same crystal structure. Here, is the lattice parameter of the solid solution, and are the lattice parameters of the pure constituents, and is the molar fraction of B in the solid solution. Vegard's law is seldom perfectly obeyed; often deviations from the linear behavior are observed. A detailed study of such deviations was conducted by King. Howe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kopp's Law
Kopp's law can refer to either of two relationships discovered by the German chemist Hermann Franz Moritz Kopp (1817–1892). #Kopp found "that the molecular heat capacity of a solid compound is the sum of the atomic heat capacities of the elements composing it; the elements having atomic heat capacities lower than those required by the Dulong–Petit law retain these lower values in their compounds." #In studying organic compounds, Kopp found a regular relationship between boiling points and the number of CH2 groups present.See page 942 of Kopp–Neumann law The Kopp–Neumann law, named for Kopp and Franz Ernst Neumann, is a common approach for determining the specific heat ''C'' (in J·kg−1·K−1) of compounds using the following equation: C = \sum_^N C_i f_i, where ''N'' is the total number of compound constituents, and ''Ci'' and ''fi'' denote the specific heat and mass fraction of the ''i''-th constituent. This law works surprisingly well at room-temperature conditi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Fraction (chemistry)
In chemistry, the mass fraction of a substance within a mixture is the ratio w_i (alternatively denoted Y_i) of the mass m_i of that substance to the total mass m_\text of the mixture. Expressed as a formula, the mass fraction is: : w_i = \frac . Because the individual masses of the ingredients of a mixture sum to m_\text, their mass fractions sum to unity: : \sum_^ w_i = 1. Mass fraction can also be expressed, with a denominator of 100, as percentage by mass (in commercial contexts often called ''percentage by weight'', abbreviated ''wt%''; see mass versus weight). It is one way of expressing the composition of a mixture in a dimensionless size; mole fraction (percentage by moles, mol%) and volume fraction ( percentage by volume, vol%) are others. When the prevalences of interest are those of individual chemical elements, rather than of compounds or other substances, the term ''mass fraction'' can also refer to the ratio of the mass of an element to the total mass of a sampl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kopp's Law
Kopp's law can refer to either of two relationships discovered by the German chemist Hermann Franz Moritz Kopp (1817–1892). #Kopp found "that the molecular heat capacity of a solid compound is the sum of the atomic heat capacities of the elements composing it; the elements having atomic heat capacities lower than those required by the Dulong–Petit law retain these lower values in their compounds." #In studying organic compounds, Kopp found a regular relationship between boiling points and the number of CH2 groups present.See page 942 of Kopp–Neumann law The Kopp–Neumann law, named for Kopp and Franz Ernst Neumann, is a common approach for determining the specific heat ''C'' (in J·kg−1·K−1) of compounds using the following equation: C = \sum_^N C_i f_i, where ''N'' is the total number of compound constituents, and ''Ci'' and ''fi'' denote the specific heat and mass fraction of the ''i''-th constituent. This law works surprisingly well at room-temperature conditi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
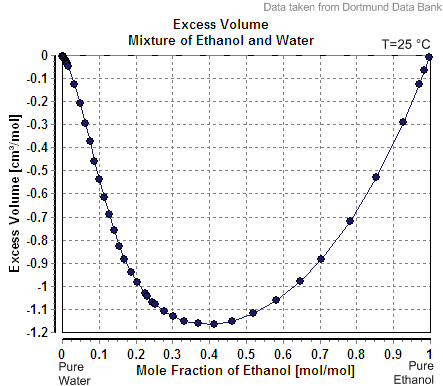
Gladstone–Dale Relation
The Gladstone–Dale relation is a mathematical relation used for optical analysis of liquids, the determination of composition from optical measurements. It can also be used to calculate the density of a liquid for use in fluid dynamics (e.g., flow visualization). The relation has also been used to calculate refractive index of glass and minerals in optical mineralogy. Uses In the Gladstone–Dale relation, (n-1)/\rho = \sum km, the index of refraction (n) or the density (ρ in g/cm3) of miscible liquids that are mixed in mass fraction (m) can be calculated from characteristic optical constants (the molar refractivity k in cm3/g) of pure molecular end-members. For example, for any mass (m) of ethanol added to a mass of water, the alcohol content is determined by measuring density or index of refraction (Brix refractometer). Mass (m) per unit volume (V) is the density m/V. Mass is conserved on mixing, but the volume of 1 cm3 of ethanol mixed with 1 cm3 of water is reduc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amagat's Law
Amagat's law or the Law of Partial Volumes describes the behaviour and properties of mixtures of ideal (as well as some cases of non-ideal) gases. It is of use in chemistry and thermodynamics. It is named after Emile Amagat. Overview Amagat's law states that the extensive volume ''V = Nv'' of a gas mixture is equal to the sum of volumes ''Vi'' of the ''K'' component gases, if the temperature ''T'' and the pressure ''p'' remain the same: : N\, v(T, p) = \sum_^K N_i\, v_i(T, p). This is the experimental expression of volume as an extensive quantity. According to Amagat's law of partial volume, the total volume of a non-reacting mixture of gases at constant temperature and pressure should be equal to the sum of the individual partial volumes of the constituent gases. So if V_1, V_2, \dots, V_n are considered to be the partial volumes of components in the gaseous mixture, then the total volume V would be represented as: :V = V_1 + V_2 + V_3 + \dots + V_n = \sum_ V_i Both Amagat's ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
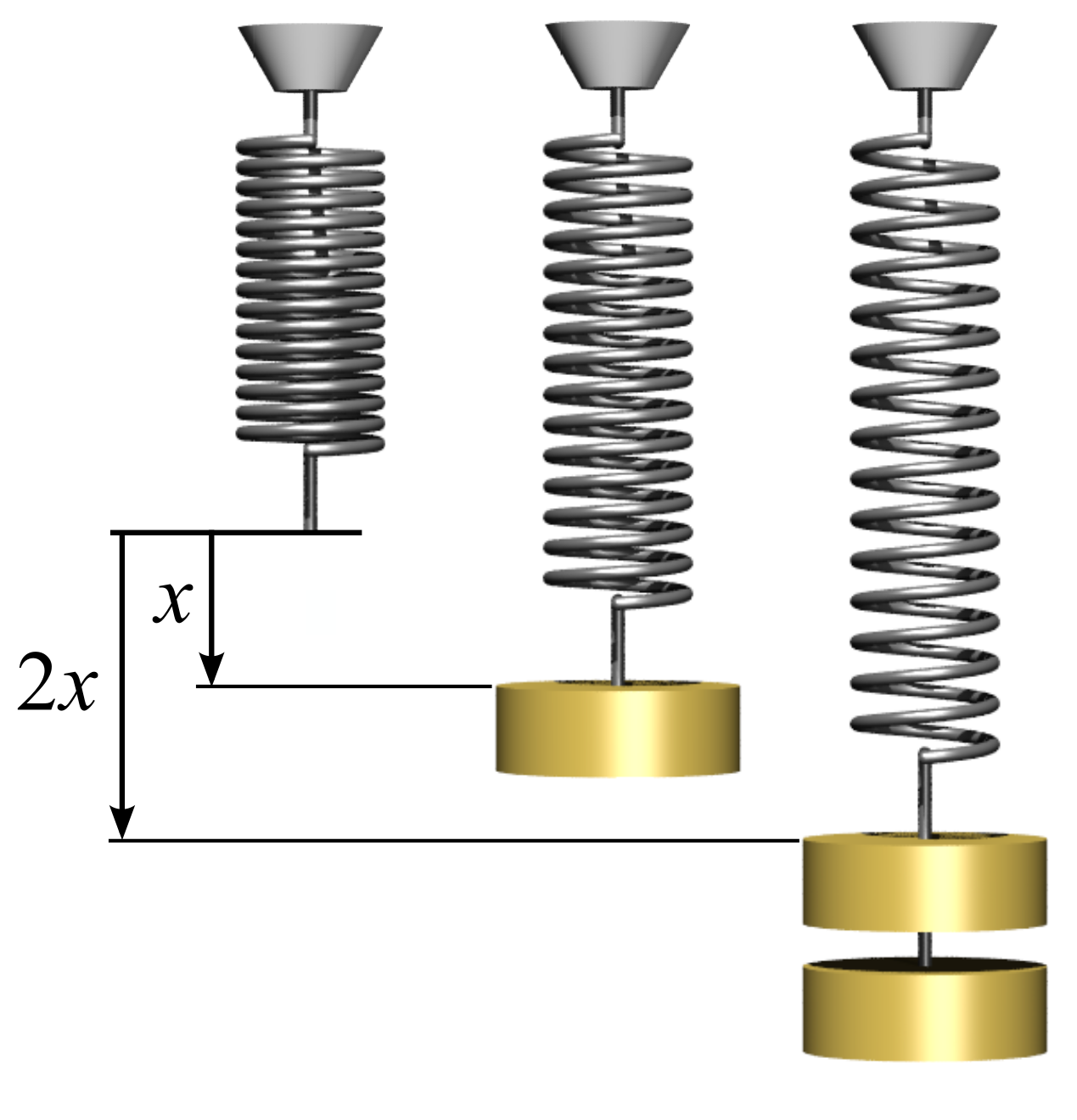
Hooke's Law
In physics, Hooke's law is an empirical law which states that the force () needed to extend or compress a spring (device), spring by some distance () Proportionality (mathematics)#Direct_proportionality, scales linearly with respect to that distance—that is, where is a constant factor characteristic of the spring (i.e., its stiffness), and is small compared to the total possible deformation of the spring. The law is named after 17th-century British physicist Robert Hooke. He first stated the law in 1676 as a Latin anagram. He published the solution of his anagram in 1678 as: ("as the extension, so the force" or "the extension is proportional to the force"). Hooke states in the 1678 work that he was aware of the law since 1660. Hooke's equation holds (to some extent) in many other situations where an elasticity (physics), elastic body is Deformation (physics), deformed, such as wind blowing on a tall building, and a musician plucking a string (music), string of a guitar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tension (physics)
In physics, tension is described as the pulling force transmitted axially by the means of a string, a rope, chain, or similar object, or by each end of a rod, truss member, or similar three-dimensional object; tension might also be described as the action-reaction pair of forces acting at each end of said elements. Tension could be the opposite of compression (physics), compression. At the atomic level, when atoms or molecules are pulled apart from each other and gain potential energy with a restoring force still existing, the restoring force might create what is also called tension. Each end of a string or rod under such tension could pull on the object it is attached to, in order to restore the string/rod to its relaxed length. Tension (as a transmitted force, as an action-reaction pair of forces, or as a restoring force) is measured in newton (unit), newtons in the International System of Units (or pounds-force in Imperial units). The ends of a string or other object transmitt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrical Conductivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allows electric current. Resistivity is commonly represented by the Greek letter (rho). The SI unit of electrical resistivity is the ohm-meter (Ω⋅m). For example, if a solid cube of material has sheet contacts on two opposite faces, and the resistance between these contacts is , then the resistivity of the material is . Electrical conductivity or specific conductance is the reciprocal of electrical resistivity. It represents a material's ability to conduct electric current. It is commonly signified by the Greek letter ( sigma), but ( kappa) (especially in electrical engineering) and ( gamma) are sometimes used. The SI unit of electrical conductivity is siemens per metre (S/m). Resistivity and conductivity are inte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |