|
Rotaxane
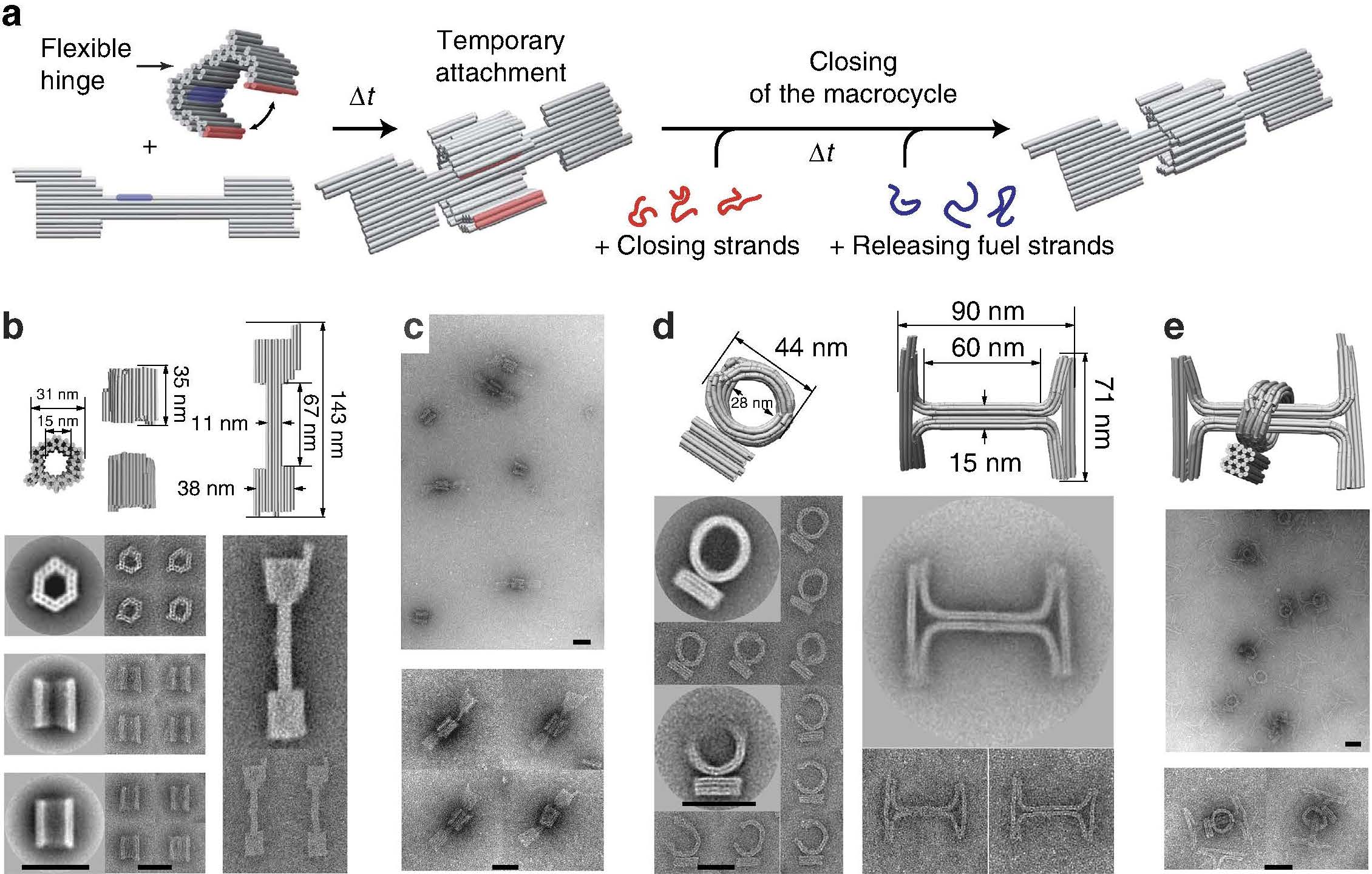
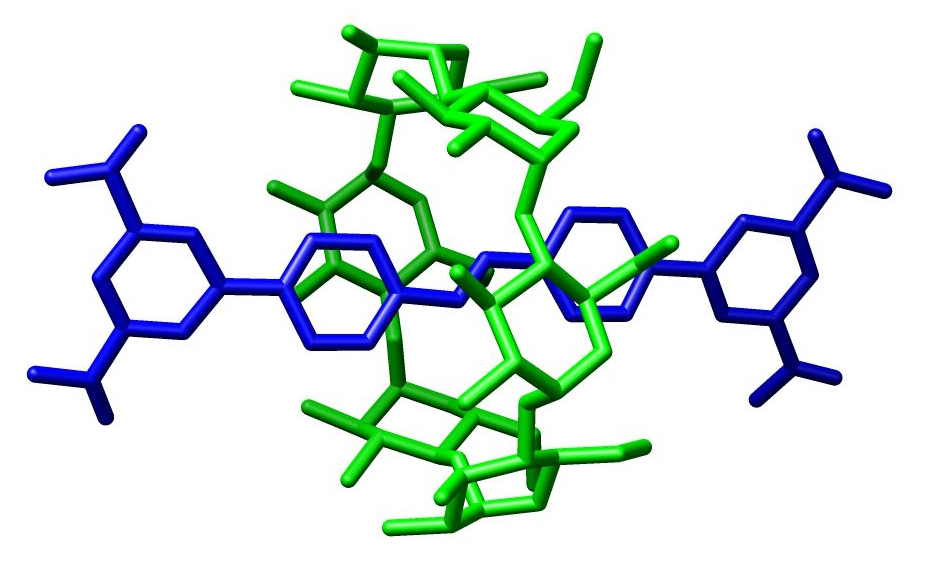
In chemistry, a rotaxane () is a mechanically interlocked molecular architecture consisting of a dumbbell-shaped molecule which is threaded through a macrocycle (see graphical representation). The two components of a rotaxane are kinetically trapped since the ends of the dumbbell (often called ''stoppers'') are larger than the internal diameter of the ring and prevent dissociation (unthreading) of the components since this would require significant distortion of the covalent bonds. Much of the research concerning rotaxanes and other mechanically interlocked molecular architectures, such as catenanes, has been focused on their efficient synthesis or their utilization as artificial molecular machines. However, examples of rotaxane substructure have been found in naturally occurring peptides, including: cystine knot peptides, cyclotides or lasso-peptides such as microcin J25. Synthesis The earliest reported synthesis of a rotaxane in 1967 relied on the statistical probabi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rotaxane Cartoon
In chemistry, a rotaxane () is a mechanically interlocked molecular architecture consisting of a dumbbell-shaped molecule which is threaded through a macrocycle (see graphical representation). The two components of a rotaxane are kinetically trapped since the ends of the dumbbell (often called ''stoppers'') are larger than the internal diameter of the ring and prevent dissociation (unthreading) of the components since this would require significant distortion of the covalent bonds. Much of the research concerning rotaxanes and other mechanically interlocked molecular architectures, such as catenanes, has been focused on their efficient synthesis or their utilization as artificial molecular machines. However, examples of rotaxane substructure have been found in naturally occurring peptides, including: cystine knot peptides, cyclotides or lasso-peptides such as microcin J25. Synthesis The earliest reported synthesis of a rotaxane in 1967 relied on the statistical probabili ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rotaxane Crystal Structure EurJOrgChem Page2565 Year1998
In chemistry, a rotaxane () is a mechanically interlocked molecular architecture consisting of a dumbbell-shaped molecule which is threaded through a macrocycle (see graphical representation). The two components of a rotaxane are kinetically trapped since the ends of the dumbbell (often called ''stoppers'') are larger than the internal diameter of the ring and prevent dissociation (unthreading) of the components since this would require significant distortion of the covalent bonds. Much of the research concerning rotaxanes and other mechanically interlocked molecular architectures, such as catenanes, has been focused on their efficient synthesis or their utilization as artificial molecular machines. However, examples of rotaxane substructure have been found in naturally occurring peptides, including: cystine knot peptides, cyclotides or lasso-peptides such as microcin J25. Synthesis The earliest reported synthesis of a rotaxane in 1967 relied on the statistical probabilit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mechanically Interlocked Molecular Architectures
In chemistry, mechanically-interlocked molecular architectures (MIMAs) are molecules that are connected as a consequence of their topology. This connection of molecules is analogous to keys on a keychain loop. The keys are not directly connected to the keychain loop but they cannot be separated without breaking the loop. On the molecular level, the interlocked molecules cannot be separated without the breaking of the covalent bonds that comprise the conjoined molecules; this is referred to as a mechanical bond. Examples of mechanically interlocked molecular architectures include catenanes, rotaxanes, molecular knots, and molecular Borromean rings. Work in this area was recognized with the 2016 Nobel Prize in Chemistry to Bernard L. Feringa, Jean-Pierre Sauvage, and J. Fraser Stoddart. The synthesis of such entangled architectures has been made efficient by combining supramolecular chemistry with traditional covalent synthesis, however mechanically interlocked molecular archite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Machine
A molecular machine, nanite, or nanomachine is a molecular component that produces quasi-mechanical movements (output) in response to specific stimuli (input). In cellular biology, macromolecular machines frequently perform tasks essential for life, such as DNA replication and ATP synthesis. The expression is often more generally applied to molecules that simply mimic functions that occur at the macroscopic level. The term is also common in nanotechnology where a number of highly complex molecular machines have been proposed that are aimed at the goal of constructing a molecular assembler. For the last several decades, chemists and physicists alike have attempted, with varying degrees of success, to miniaturize machines found in the macroscopic world. Molecular machines are at the forefront of cellular biology research. The 2016 Nobel Prize in Chemistry was awarded to Jean-Pierre Sauvage, Sir J. Fraser Stoddart, and Bernard L. Feringa for the design and synthesis of molecula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Switch
A molecular switch is a molecule that can be reversibly shifted between two or more stable states. The molecules may be shifted between the states in response to environmental stimuli, such as changes in pH, light, temperature, an electric current, microenvironment, or in the presence of ions and other ligands. In some cases, a combination of stimuli is required. The oldest forms of synthetic molecular switches are pH indicators, which display distinct colors as a function of pH. Currently synthetic molecular switches are of interest in the field of nanotechnology for application in molecular computers or responsive drug delivery systems. Molecular switches are also important in biology because many biological functions are based on it, for instance allosteric regulation and vision. They are also one of the simplest examples of molecular machines. Biological molecular switches In cellular biology, proteins act as intracellular signaling molecules by activating another protein i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catenane
In macromolecular chemistry, a catenane () is a mechanically interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be separated without breaking the covalent bonds of the macrocycles. They are conceptually related to other mechanically interlocked molecular architectures, such as rotaxanes, molecular knots or molecular Borromean rings. Recently the terminology "mechanical bond" has been coined that describes the connection between the macrocycles of a catenane. Catenanes have been synthesised in two different ways: statistical synthesis and template-directed synthesis. Synthesis There are two primary approaches to the organic synthesis of catenanes. The first is to simply perform a ring-closing reaction with the hope that some of the rings will form around other rings giving the desired catenane product. This so-called "statistical approach" led to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Shuttle
A molecular shuttle in supramolecular chemistry is a special type of molecular machine capable of shuttling molecules or ions from one location to another. This field is of relevance to nanotechnology in its quest for nanoscale electronic components and also to biology where many biochemical functions are based on molecular shuttles. Academic interest also exists for synthetic molecular shuttles, the first prototype reported in 1991 based on a rotaxane. This device is based on a molecular thread composed of an ethyleneglycol chain interrupted by two arene groups acting as so-called stations. The terminal units (or stoppers) on this wire are bulky triisopropylsilyl groups. The bead is a tetracationic cyclophane based on two bipyridine groups and two para-phenylene groups. The bead is locked to one of the stations by pi-pi interactions but since the activation energy for migration from one station to the other station is only 13 kcal/ mol (54 kJ/mol) the bead shuttles between the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Electronics
Molecular electronics is the study and application of molecular building blocks for the fabrication of electronic components. It is an interdisciplinary area that spans physics, chemistry, and materials science. The unifying feature is use of molecular building blocks to fabricate electronic components. Due to the prospect of size reduction in electronics offered by molecular-level control of properties, molecular electronics has generated much excitement. It provides a potential means to extend Moore's Law beyond the foreseen limits of small-scale conventional silicon integrated circuits. Molecular scale electronics Molecular scale electronics, also called single-molecule electronics, is a branch of nanotechnology that uses single molecules, or nanoscale collections of single molecules, as electronic components. Because single molecules constitute the smallest stable structures possible, this miniaturization is the ultimate goal for shrinking electrical circuits. Conventio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystine Knot
A cystine knot is a protein structural motif containing three disulfide bridges (formed from pairs of cysteine residues). The sections of polypeptide that occur between two of them form a loop through which a third disulfide bond passes, forming a rotaxane substructure. The cystine knot motif stabilizes protein structure and is conserved in proteins across various species. There are three types of cystine knot, which differ in the topology of the disulfide bonds: * The growth factor cystine knot (GFCK) * inhibitor cystine knot (ICK) common in spider and snail toxins * Cyclic Cystine Knot, or cyclotide The growth factor cystine knot was first observed in the structure of nerve growth factor (NGF), solved by X-ray crystallography and published in 1991 by Tom Blundell in Nature.; The GFCK is present in four superfamilies. These include nerve growth factor, transforming growth factor beta (TGF-β), platelet-derived growth factor, and glycoprotein hormones including human chorioni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chem
Chem may refer to: * Chemistry practical waali mam * Chemistry *Chemical * ''Chem'' (journal), a scientific journal published by Cell Press *Post apocalyptic slang for "drugs", medicinal or otherwise in the Fallout video game series. In Ancient Egyptian usage: * ''Khem'' (also spelt ''Chem''), the Egyptian word for "black" *Min (god), in the past erroneously named ''Khem'' CHEM may refer to : *A metabolic panel: for instance, CHEM-7, which is the basic metabolic panel * CHEM-DT, a Canadian television channel See also *Chemo (other) Chemo is a prefix meaning ''chemical'' and commonly used as an abbreviation for chemotherapy. Chemo may also refer to: People * Chemo (musician), an English musician now known as Forest DLG * Chemo Soto, a Puerto Rican politician * José del Sol ... * Kemi, a place in Finland {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |