|
Propargyl Group
In organic chemistry, the propargyl group is a functional group of 2-propynyl with the structure . It is an alkyl group derived from propyne (). The term propargylic refers to a saturated position ( ''sp''3-hybridized) on a molecular framework next to an alkynyl group. The name comes from mix of ''propene'' and ''argentum'', which refers to the typical reaction of the terminal alkynes with silver salts. The term homopropargylic designates in the same manner * a saturated position on a molecular framework next to a propargylic group and thus two bonds from an alkyne moiety. * a 3-butynyl fragment, , or substituted homologue. See also * Alkenyl groups ** Allyl ** Vinyl group * Ethynyl * Propargyl chloride * Propargyl alcohol * Propargyl bromide * Propiolic acid Propiolic acid is the organic compound with the formula HC2CO2H. It is the simplest acetylenic carboxylic acid. It is a colourless liquid that crystallises to give silky crystals. Near its boiling point, it decompos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propargyl
In organic chemistry, the propargyl group is a functional group of 2-propynyl with the structure . It is an alkyl group derived from propyne (). The term propargylic refers to a saturated position ( ''sp''3-hybridized) on a molecular framework next to an alkynyl group. The name comes from mix of ''propene'' and ''argentum'', which refers to the typical reaction of the terminal alkynes with silver salts. The term homopropargylic designates in the same manner * a saturated position on a molecular framework next to a propargylic group and thus two bonds from an alkyne moiety. * a 3-butynyl fragment, , or substituted homologue. See also * Alkenyl groups ** Allyl ** Vinyl group * Ethynyl * Propargyl chloride * Propargyl alcohol * Propargyl bromide * Propiolic acid Propiolic acid is the organic compound with the formula HC2CO2H. It is the simplest acetylenic carboxylic acid. It is a colourless liquid that crystallises to give silky crystals. Near its boiling point, it decompo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synlett
''Synlett'' is an international scientific journal for accounts and rapid communications of original contributions of fundamental research in synthetic organic chemistry. The impact factor of this journal is 2.419 (2017). ''Nature'' featured a brief piece by the editor-in-chief of the journal in 2017, Benjamin List Benjamin ( he, ''Bīnyāmīn''; "Son of (the) right") blue letter bible: https://www.blueletterbible.org/lexicon/h3225/kjv/wlc/0-1/ H3225 - yāmîn - Strong's Hebrew Lexicon (kjv) was the last of the two sons of Jacob and Rachel (Jacob's thir ..., where he discussed the journal's experience with the non-traditional peer review system. References Chemistry journals Thieme academic journals Publications established in 1989 {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
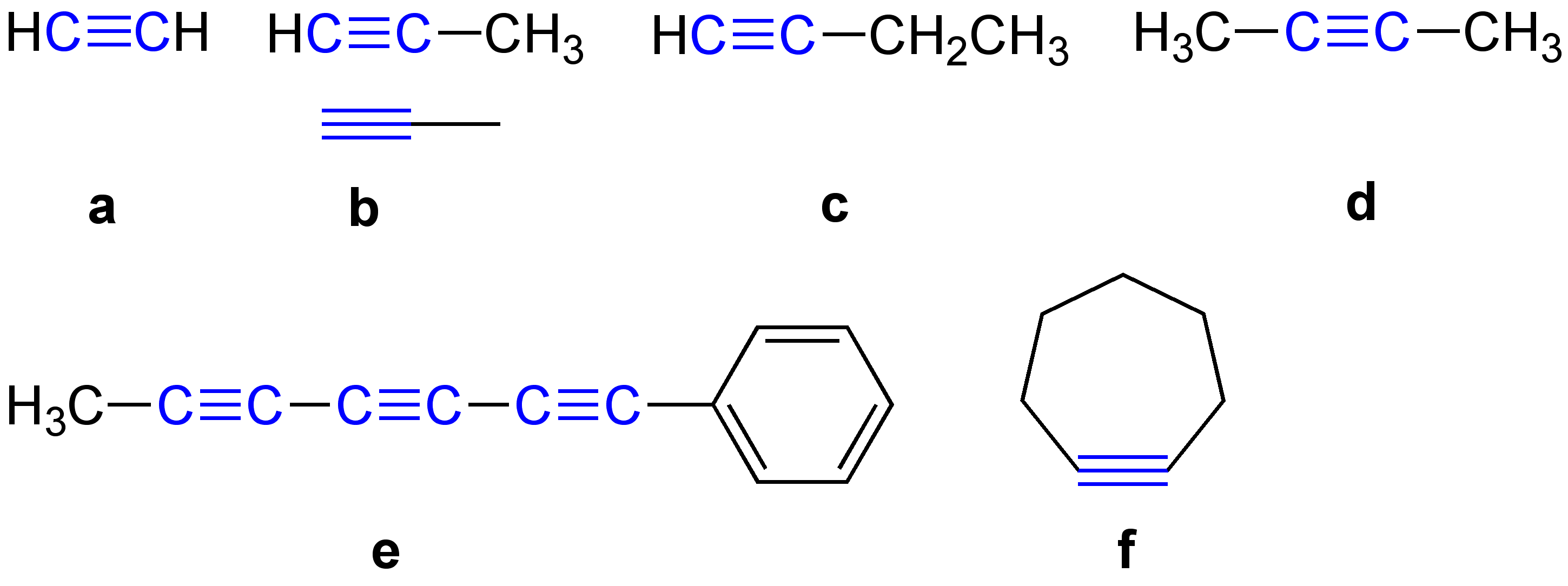
Alkynyl Groups
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes (134 pm) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contributes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propiolic Acid
Propiolic acid is the organic compound with the formula HC2CO2H. It is the simplest acetylenic carboxylic acid. It is a colourless liquid that crystallises to give silky crystals. Near its boiling point, it decomposes. It is soluble in water and possesses an odor like that of acetic acid. Preparation It is prepared commercially by oxidizing propargyl alcohol at a lead electrode. It can also be prepared by decarboxylation of acetylenedicarboxylic acid. : Reactions and applications Exposure to sunlight converts it into trimesic acid (benzene-1,3,5-tricarboxylic acid). It undergoes bromination to give dibromoacrylic acid. With hydrogen chloride it forms chloroacrylic acid. Its ethyl ester condenses with hydrazine to form pyrazolone. It forms a characteristic explosive solid upon treatment to its aqueous solution with ammoniacal silver nitrate. An amorphous explosive precipitate forms with ammoniacal cuprous chloride. Propiolates Propiolates are esters or salts of propiolic a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propargyl Bromide
Propargyl bromide, also known as 3-bromo-prop-1-yne, is an organic compound with the chemical formula HC≡CCH2Br. A colorless liquid, it is a halogenated organic compound consisting of propyne with a bromine substituent on the methyl group. It has a lachrymatory effect, like related compounds. The compound is used as a reagent in organic synthesis. Applications and production Propargyl bromide can also be used as an intermediate for the synthesis of organic compounds, including agrochemicals and pharmaceuticals. In the 1960s, it was used in a soil fumigant called Trizone.Franz Müller and Arnold P. Applebyki "Weed Control, 2. Individual Herbicides" in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2010, Propargyl bromide may be produced by the treatment of propargyl alcohol with phosphorus tribromide. Reactions Propargyl bromide is an alkylating agent. With dimethylsulfide, it reacts to give the sulfonium salt: : It also alkylates even weakly basic amines such as ani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propargyl Alcohol
Propargyl alcohol, or 2-propyn-1-ol, is an organic compound with the chemical formula, formula C3H4O. It is the simplest stable Alcohol (chemistry), alcohol containing an alkyne functional group. Propargyl alcohol is a colorless viscous liquid that is miscible with water and most polar organic solvents. Reactions and applications Propargyl alcohol polymerizes with heating or treatment with base (chemistry), base. It is used as a corrosion inhibitor, a metal complex solution, a solvent stabilizer and an electroplating brightener additive. It is also used as an intermediate in organic synthesis. Secondary alcohol, Secondary and Tertiary alcohol, tertiary substituted propargylic alcohols undergo catalyzed rearrangement reactions to form carbonyl#α,β-Unsaturated carbonyl compounds, α,β-unsaturated carbonyl compounds via the Meyer–Schuster rearrangement and others. It can be oxidized to propynal or propargylic acid. As an indication of the electronegativity of an Orbital hybri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propargyl Chloride
Propargyl chloride is an organic compound with the formula HC2CH2Cl. It is a colorless liquid and a lacrymator. It is an alkylating agent that is used in organic synthesis. See also * Allyl chloride * Propargyl * Propargyl alcohol Propargyl alcohol, or 2-propyn-1-ol, is an organic compound with the chemical formula, formula C3H4O. It is the simplest stable Alcohol (chemistry), alcohol containing an alkyne functional group. Propargyl alcohol is a colorless viscous liquid t ... References External links Entry at ChemSpider Propargyl compounds Organochlorides {{organohalide-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethynyl Radical
The ethynyl radical (systematically named λ3-ethyne and hydridodicarbon(''C''—''C'')) is an organic compound with the chemical formula C≡CH (also written CHor ). It is a simple molecule that does not occur naturally on Earth but is abundant in the interstellar medium. It was first observed by electron spin resonance isolated in a solid argon matrix at liquid helium temperatures in 1963 by Cochran and coworkers at the Johns Hopkins Applied Physics Laboratory. It was first observed in the gas phase by Tucker and coworkers in November 1973 toward the Orion Nebula, using the NRAO 11-meter radio telescope. It has since been detected in a large variety of interstellar environments, including dense molecular clouds, bok globules, star forming regions, the shells around carbon-rich evolved stars, and even in other galaxies. Astronomical Importance Observations of C2H can yield a large number of insights into the chemical and physical conditions where it is located. First, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinyl Group
In organic chemistry, a vinyl group (abbr. Vi; IUPAC name: ethenyl group) is a functional group with the formula . It is the ethylene (IUPAC name: ethene) molecule () with one fewer hydrogen atom. The name is also used for any compound containing that group, namely where R is any other group of atoms. An industrially important example is vinyl chloride, precursor to PVC, a plastic commonly known as ''vinyl''. Vinyl is one of the alkenyl functional groups. On a carbon skeleton, sp2-hybridized carbons or positions are often called vinylic. Allyls, acrylates and styrenics contain vinyl groups. (A styrenic crosslinker with two vinyl groups is called '' divinyl benzene''.) Vinyl polymers Vinyl groups can polymerize with the aid of a radical initiator or a catalyst, forming vinyl polymers. Vinyl polymers contain no vinyl groups. Instead they are saturated. The following table gives some examples of vinyl polymers. Reactivity Vinyl derivatives are alkenes. If activated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes (134 pm) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contribute ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |