|
Proline Organocatalysis
Proline organocatalysis is the use of proline as an organocatalyst in organic chemistry. This theme is often considered the starting point for the area of organocatalysis, even though early discoveries went unappreciated. Modifications, such as MacMillan’s catalyst and Jorgensen's catalysts, proceed with excellent stereocontrol. Proline catalysis was initially reported by groups at Schering AG and Hoffmann-La Roche. Proline's chiral structure enables enantioselective synthesis, favoring a particular enantiomer or diastereomer. Reactions The Hajos–Parrish–Eder–Sauer–Wiechert reaction, reported in 1971 by several research teams, is an early example of an enantioselective catalytic reaction in organic chemistry. Its scope has been modified and expanded through the development of related reactions including the Michael addition, asymmetric aldol reaction, and the Mannich reaction. This reaction has likewise been used to perform asymmetric Robinson annulations. The gener ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG). Proline is the only proteinogenic secondary amino acid which is a secondary amine, as the nitrogen atom is attached both to the α-carbon and to a chain of three carbons that together form a five-membered ring. History and etymology Proline was first isolated in 1900 by Richard Willstätter who obtained the amino ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
H-bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted , where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are the second-row elements nitrogen (N), oxygen (O), and fluorine (F). Hydrogen bonds can be intermolecular (occurring between separate molecules) or intramolecular (occurring among parts of the same molecule). The energy of a hydrogen bond depends on the geometry, the environment, and the nature of the specific donor and acceptor atoms and can vary between 1 and 40 kcal/mol. This makes them somewhat stronger than a van der Waals interaction, and weaker than fully covalent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyacetone
Hydroxyacetone, also known as acetol, is the organic chemical with the formula CH3C(O)CH2OH. It consists of a primary alcohol substituent on acetone. It is an α-hydroxyketone, also called a ketol, and is the simplest hydroxy ketone structure. It is a colorless, distillable liquid. Preparation It is produced commercially by dehydration of glycerol. Hydroxyacetone is commercially available, but it also may be synthesized on a laboratory scale by a substitution reaction on bromoacetone. Reactions It undergoes rapid polymerization, including forming a hemiacetal cyclic dimer. Under alkaline conditions, it undergoes a rapid aldol condensation. Hydroxyacetone can be produced by degradation of various sugars. In foods, it is formed by the Maillard reaction. It reacts further to form other compounds with various aromas. As such it finds some use as a flavoring. See also * Acyloin Acyloins or α-hydroxy ketones are a class of organic compounds which all possess a hydroxy group a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour. Acetone is miscible with water and serves as an important organic solvent in its own right, in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and production of methyl methacrylate (and from that PMMA) as well as bisphenol A.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in |
(R)-2-Methyl-CBS-oxazaborolidine
(''R'')-2-Methyl-CBS-oxazaborolidine is an organoboron catalyst that is used in organic synthesis. This catalyst, developed by Itsuno and Elias James Corey, is generated by heating (''R'')-(+)-2-(diphenylhydroxymethyl) pyrrolidine along with trimethylboroxine or methylboronic acid. It is an excellent tool for the synthesis of alcohols in high enantiomeric ratio. Generally, 2-10 mol% of this catalyst is used along with borane-tetrahydrofuran (THF), borane-dimethylsulfide, borane- ''N'',''N''-diethylaniline, or diborane as the borane source. Enantioselective reduction using chiral oxazaborolidine catalysts has been used in the synthesis of commercial drugs such as ezetimibe and aprepitant Aprepitant, sold under the brand name Emend among others, is a medication used to prevent chemotherapy-induced nausea and vomiting (CINV) and to prevent postoperative nausea and vomiting (PONV). It may be used together with ondansetron and de .... See also * CBS catalyst * Corey-I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iminium
In organic chemistry, an iminium cation is a polyatomic ion with the general structure . They are common in synthetic chemistry and biology. Structure Iminium cations adopt alkene-like geometries. The central C=N unit is nearly coplanar with all four substituents. The C=N distances, which are near 129 picometers in length, are shorter than C-N single bonds. Cis/trans isomers are observed. Formation Iminium cations are obtained by protonation and alkylation of imines: :RN=CR'_2 + H+ -> NH=CR'_2 :RN=CR'_2 + R''+ -> R''N=CR'_2 They also are generated by the condensation of secondary amines with ketones or aldehydes: :O=CR'_2 + R2NH + H+ 2N=CR'_2 + H2O This rapid, reversible reaction is one step in "iminium catalysis". More exotic routes to iminium cations are known, e.g. from ring-opening reactions of pyridine. Occurrence Iminium derivatives are common in biology. Pyridoxal phosphate reacts with amino acids to give iminium derivatives. Many iminium salts are en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
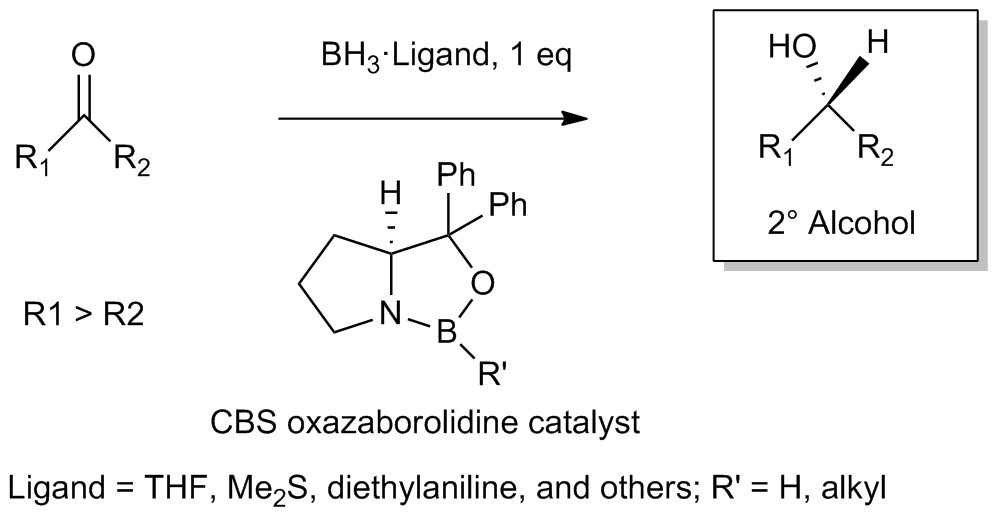
Corey–Itsuno Reduction
The Corey–Itsuno reduction, also known as the Corey–Bakshi–Shibata (CBS) reduction, is a chemical reaction in which an achiral ketone is enantioselectively reduced to produce the corresponding chiral, non-racemic alcohol. The oxazaborolidine reagent which mediates the enantioselective reduction of ketones was previously developed by the laboratory of Itsuno and thus this transformation may more properly be called the Itsuno-Corey oxazaborolidine reduction. History In 1981, Itsuno and coworkers first reported the use of chiral alkoxy-amine-borane complexes in reducing achiral ketones to chiral alcohols enantioselectively and in high yield. Several years later in 1987, E. J. Corey and coworkers developed the reaction between chiral amino alcohols and borane (BH3), generating oxazaborolidine products which were shown to rapidly catalyze the enantioselective reduction of achiral ketones in the presence of BH3•THF. The CBS reduction has since been utilized by organic chemists ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enders SAMP/RAMP Hydrazone-alkylation Reaction
The Enders SAMP/RAMP hydrazone alkylation reaction is an asymmetric synthesis, asymmetric carbon-carbon chemical bond, bond formation reaction facilitated by pyrrolidine chiral auxiliaries. It was pioneered by E. J. Corey and Dieter Enders, D. Enders in 1976, and was further developed by Dieter Enders, D. Enders and his group. This method is usually a three-step sequence. The first step is to form the hydrazone between (''S'')-1-amino-2-methoxymethylpyrrolidine (SAMP) or (''R'')-1-amino-2-methoxymethylpyrrolidine (RAMP) and a ketone or aldehyde. Afterwards, the hydrazone is deprotonated by lithium diisopropylamide (lithium diisopropylamide, LDA) to form an enolate, azaenolate, which reacts with alkyl halides or other suitable electrophiles to give alkylated hydrazone species with the simultaneous generation of a new chiral center. Finally, the alkylated ketone or aldehyde can be regenerated by ozonolysis or hydrolysis. This reaction is a useful technique for asymmetric α-alkylatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michael Reaction
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds. The Michael addition is an important atom-economical method for diastereoselective and enantioselective C–C bond formation, and many asymmetric variants exist : In this general Michael addition scheme, either or both of R and R' on the nucleophile (the Michael donor) represent electron-withdrawing substituents such as acyl, cyano, nitro, or sulfone groups, which make the adjacent methylene hydrogen acidic enough to form a carbanion when reacted with the base, ''B:''. For the alkene (the Michael acceptor), the R" substituent is usually a carbonyl, which makes the compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_with_Enamine_intermediate.png)