|
Potassium Ferrocyanide
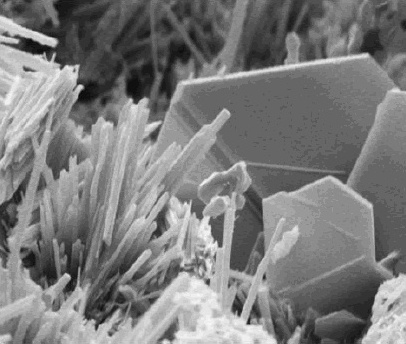
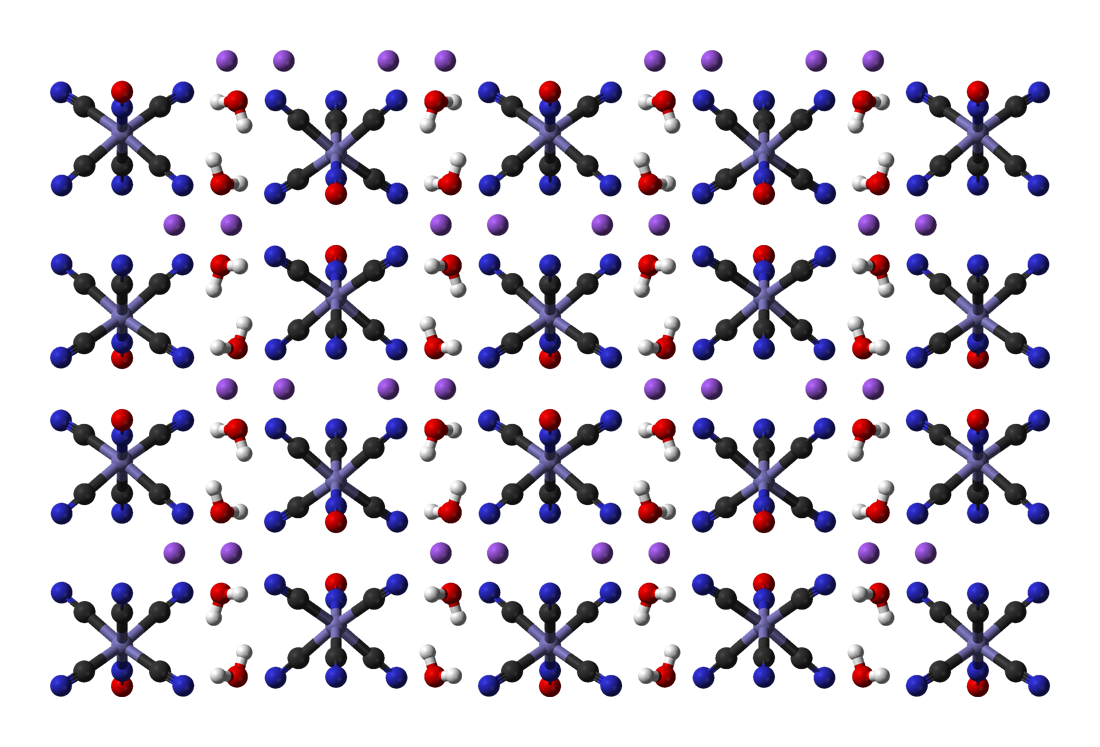
Potassium ferrocyanide is the inorganic compound with formula K4 e(CN)6ˇ3H2O. It is the potassium salt of the coordination complex e(CN)6sup>4â. This salt forms lemon-yellow monoclinic crystals. Synthesis In 1752, the French chemist Pierre Joseph Macquer (1718â1784) first reported the preparation of potassium ferrocyanide, which he achieved by reacting Prussian blue (iron(III) ferrocyanide) with potassium hydroxide. Modern production Potassium ferrocyanide is produced industrially from hydrogen cyanide, ferrous chloride, and calcium hydroxide, the combination of which affords Ca2 e(CN)6ˇ11H2O. This solution is then treated with potassium salts to precipitate the mixed calcium-potassium salt CaK2 e(CN)6 which in turn is treated with potassium carbonate to give the tetrapotassium salt. Historical production Historically, the compound was manufactured from organic compounds containing nitrogen, iron filings, and potassium carbonate. Common nitrogen and carbon sources wer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a Volatility (chemistry), volatile, Combustibility and flammability, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of Carbohydrate, sugars by yeasts or via Petrochemistry, petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the Chemical synthesis, synthesis of organic compounds, and as a Alcohol fuel, fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed or slaked with water. It has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide. Properties Calcium hydroxide is poorly soluble in water, with a retrograde solubility increasing from 0.66 g/L at 100 °C to 1.89 g/L at 0 °C. With a solubility product ''K''sp of 5.02 at 25 °C, its dissociation in water is large enough that its solutions are basic according to the following dissolution reaction: : Ca(OH)2 â Ca2+ + 2 OHâ At ambient temperature, calcium hydroxide (portlandite) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Permanganate
Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, that dissolves in water as K+ and , an intensely pink to purple solution. Potassium permanganate is widely used in the chemical industry and laboratories as a strong oxidizing agent, and also as a medication for dermatitis, for cleaning wounds, and general disinfection. It is on the World Health Organization's List of Essential Medicines. In 2000, worldwide production was estimated at 30,000 tonnes. Properties Potassium permanganate is the potassium salt of the tetrahedral transition metal oxo complex permanganate, in which four O2- ligands are bound to a manganese(VII) center. Structure KMnO4 forms orthorhombic crystals with constants: ''a'' = 910.5 pm, ''b'' = 572.0 pm, ''c'' = 742.5 pm. The overall motif is similar to that for barium sulfate, with which it forms solid solutions. In the solid (as in solution), each MnO4â centre is t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blueprint
A blueprint is a reproduction of a technical drawing or engineering drawing using a contact print process on light-sensitive sheets. Introduced by Sir John Herschel in 1842, the process allowed rapid and accurate production of an unlimited number of copies. It was widely used for over a century for the reproduction of specification drawings used in construction and industry. The blueprint process was characterized by white lines on a blue background, a negative of the original. The process was not able to reproduce color or shades of grey. The process is now obsolete. It was first largely displaced by the diazo whiteprint process, and later by large-format xerographic photocopiers. The term ''blueprint'' continues to be used less formally to refer to any floor plan (and even less formally, any type of plan). Practicing engineers, architects, and drafters often call them "drawings", âprintsâ, or âplansâ. It has almost entirely been replaced with digital computer-aided ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms. In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom. In inorganic cyanides, the cyanide group is present as the anion . Soluble salts such as sodium cyanide (NaCN) and potassium cyanide (KCN) are highly toxic. Hydrocyanic acid, also known as hydrogen cyanide, or HCN, is a highly volatile liquid that is produced on a large scale industrially. It is obtained by acidification of cyanide salts. Organic cyanides are usually called nitriles. In nitriles, the group is linked by a covalent bond to carbon. For example, in acetonitrile (), the cyanide group is bonded to methyl (). Although nitriles generally do not release cyanide ions, the cyanohydrins do and are thus rather toxic. Bonding The cyanide ion is isoelectronic with carbon monoxide a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Ferricyanide
Potassium ferricyanide is the chemical compound with the formula K3 e(CN)6 This bright red salt contains the octahedrally coordinated 3â.html" ;"title="e(CN)6sup>3â">e(CN)6sup>3â ion. It is soluble in water and its solution shows some green-yellow fluorescence. It was discovered in 1822 by Leopold Gmelin. Preparation Potassium ferricyanide is manufactured by passing chlorine through a solution of potassium ferrocyanide. Potassium ferricyanide separates from the solution: :2 K4 e(CN)6+ Cl2 â 2 K3 e(CN)6+ 2 KCl Structure Like other metal cyanides, solid potassium ferricyanide has a complicated polymeric structure. The polymer consists of octahedral e(CN)6sup>3â centers crosslinked with K+ ions that are bound to the CN ligands. The K+---NCFe linkages break when the solid is dissolved in water. Applications The compound is also used to harden iron and steel, in electroplating, dyeing wool, as a laboratory reagent, and as a mild oxidizing agent in organic chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and hydrochloric acid (in the form of ). However ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Nitroprusside
Sodium nitroprusside (SNP), sold under the brand name Nitropress among others, is a medication used to lower blood pressure. This may be done if the blood pressure is very high and resulting in symptoms, in certain types of heart failure, and during surgery to decrease bleeding. It is used by continuous injection into a vein. Onset is nearly immediate and effects last for up to ten minutes. It is available as a generic medication. Side effects and mechanism Common side effects include low blood pressure and cyanide toxicity. Other serious side effects include methemoglobinemia. It is not generally recommended during pregnancy due to concerns of side effects. High doses are not recommended for more than ten minutes. It works by increasing nitric oxide levels in the blood, which increases cGMP levels in cells, and causes dilation of blood vessels. History, society and culture Sodium nitroprusside was discovered as early as 1850 and found to be useful in medicine in 1928. It i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions in water. Historically, it was extracted from the ashes of plants growing in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process. Hydrates Sodium carbonate is obtained as three hydrates and as the anhydrous salt: * sodium carbonate decahydrate (natron), Na2CO3¡10H2O, which readily efflorescence, effloresces to form the monohydrate. * sodium carbonate heptahydrate (not known in mineral form), Na2CO3¡7H2O. * sodium carbonate monohydrate (thermonatrite), Na2CO3¡H2O. Also known as crystal carbonate. * anhydrous sodium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% , it is referred to as ''fuming nitric acid''. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%. Nitric acid is the primary reagent used for nitration â the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as pigments in inks and dyes. Nitric acid is also commonly used as a strong oxidizing agen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Offal
Offal (), also called variety meats, pluck or organ meats, is the organs of a butchered animal. The word does not refer to a particular list of edible organs, which varies by culture and region, but usually excludes muscle. Offal may also refer to the by-products of milled grains, such as corn or wheat. Some cultures strongly consider offal as food to be taboo, while others use it as everyday food or even as delicacies. Certain offal dishesâincluding '' foie gras'', '' pâtĂŠ'', and haggis âare internationally regarded as gourmet food in the culinary arts. Others remain part of traditional regional cuisine and may be consumed especially during holidays. This includes sweetbread, Jewish chopped liver, U.S. chitterlings, Mexican menudo, as well as many other dishes. On the other hand, intestines are traditionally used as casing for sausages. Depending on the context, ''offal'' may refer only to those parts of an animal carcass discarded after butchering or skinning ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Torrefaction
Torrefaction of biomass, e.g., wood or grain, is a mild form of pyrolysis at temperatures typically between 200 and 320 °C. Torrefaction changes biomass properties to provide a better fuel quality for combustion and gasification applications. Torrefaction produces a relatively dry product, which reduces or eliminates its potential for organic decomposition. Torrefaction combined with densification creates an energy-dense fuel carrier of 20 to 21 GJ/ton lower heating value (LHV). Torrefaction makes the material undergo Maillard reactions. Torrefied biomass can be used as an energy carrier or as a feedstock used in the production of bio-based fuels and chemicals. Biomass can be an important energy source. However, there exists a large diversity of potential biomass sources, each with its own unique characteristics. To create efficient biomass-to-energy chains, torrefaction of biomass, combined with densification ( pelletisation or briquetting), is a promising step toward ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |