|
Polytropic
A polytropic process is a thermodynamic process that obeys the relation: p V^ = C where ''p'' is the pressure, ''V'' is volume, ''n'' is the polytropic index, and ''C'' is a constant. The polytropic process equation describes expansion and compression processes which include heat transfer. Particular cases Some specific values of ''n'' correspond to particular cases: * n=0 for an isobaric process, * n=+\infty for an isochoric process. In addition, when the ideal gas law applies: * n=1 for an isothermal process, * n=\gamma for an isentropic process. Where \gamma is the ratio of the heat capacity at constant pressure (C_P) to heat capacity at constant volume (C_V). Equivalence between the polytropic coefficient and the ratio of energy transfers For an ideal gas in a closed system undergoing a slow process with negligible changes in kinetic and potential energy the process is polytropic, such that p v^ = C where ''C'' is a constant, K = \frac, \gamma = \frac, and with t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polytropic
A polytropic process is a thermodynamic process that obeys the relation: p V^ = C where ''p'' is the pressure, ''V'' is volume, ''n'' is the polytropic index, and ''C'' is a constant. The polytropic process equation describes expansion and compression processes which include heat transfer. Particular cases Some specific values of ''n'' correspond to particular cases: * n=0 for an isobaric process, * n=+\infty for an isochoric process. In addition, when the ideal gas law applies: * n=1 for an isothermal process, * n=\gamma for an isentropic process. Where \gamma is the ratio of the heat capacity at constant pressure (C_P) to heat capacity at constant volume (C_V). Equivalence between the polytropic coefficient and the ratio of energy transfers For an ideal gas in a closed system undergoing a slow process with negligible changes in kinetic and potential energy the process is polytropic, such that p v^ = C where ''C'' is a constant, K = \frac, \gamma = \frac, and with t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
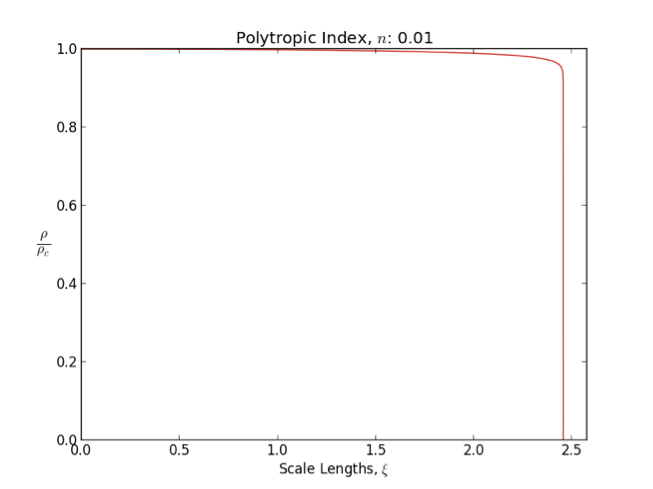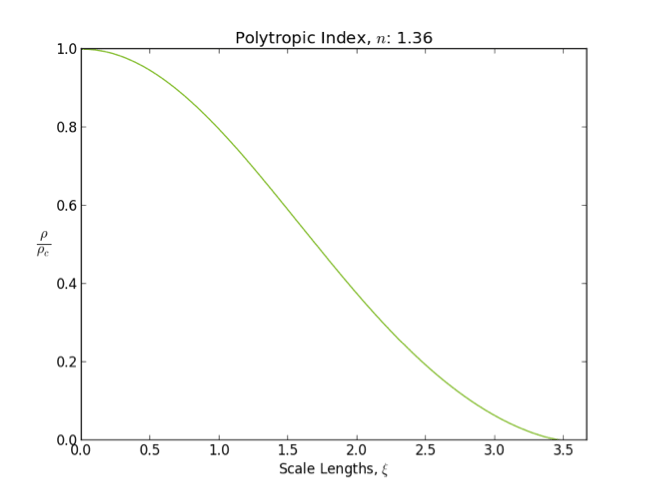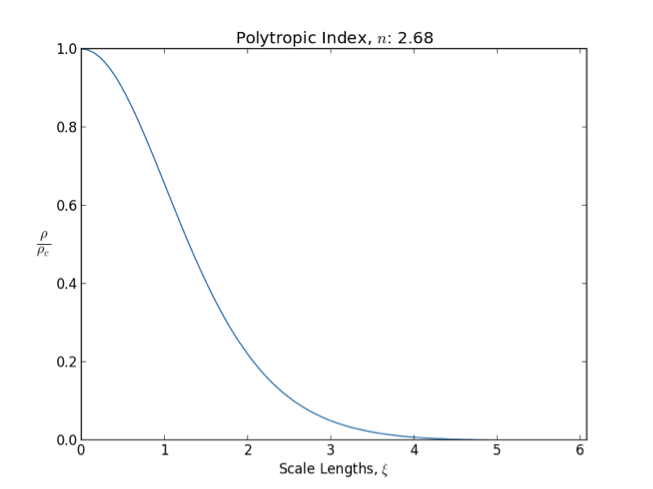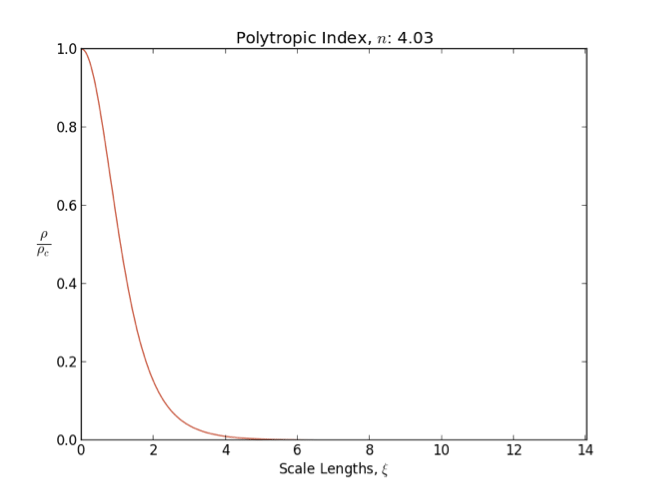
Lane–Emden Equation
In astrophysics, the Lane–Emden equation is a dimensionless form of Poisson's equation for the gravitational potential of a Newtonian self-gravitating, spherically symmetric, polytropic fluid. It is named after astrophysicists Jonathan Homer Lane and Robert Emden. The equation reads : \frac \frac \left(\right) + \theta^n = 0, where \xi is a dimensionless radius and \theta is related to the density, and thus the pressure, by \rho=\rho_c\theta^n for central density \rho_c. The index n is the polytropic index that appears in the polytropic equation of state, : P = K \rho^\, where P and \rho are the pressure and density, respectively, and K is a constant of proportionality. The standard boundary conditions are \theta(0)=1 and \theta'(0)=0. Solutions thus describe the run of pressure and density with radius and are known as ''polytropes'' of index n. If an isothermal fluid (polytropic index tends to infinity) is used instead of a polytropic fluid, one obtains the Emden–Chan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Compressor
A compressor is a mechanical device that increases the pressure of a gas by reducing its volume. An air compressor is a specific type of gas compressor. Compressors are similar to pumps: both increase the pressure on a fluid and both can transport the fluid through a pipe. The main distinction is that the focus of a compressor is to change the density or volume of the fluid, which is mostly only achievable on gases. Gases are compressible, while liquids are relatively incompressible, so compressors are rarely used for liquids. The main action of a pump is to pressurize and transport liquids. Many compressors can be staged, that is, the fluid is compressed several times in steps or stages, to increase discharge pressure. Often, the second stage is physically smaller than the primary stage, to accommodate the already compressed gas without reducing its pressure. Each stage further compresses the gas and increases its pressure and also temperature (if inter cooling between stages i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polytrope
In astrophysics, a polytrope refers to a solution of the Lane–Emden equation in which the pressure depends upon the density in the form :P = K \rho^, where is pressure, is density and is a constant of proportionality. The constant is known as the polytropic index; note however that the polytropic index has an alternative definition as with ''n'' as the exponent. This relation need not be interpreted as an equation of state, which states ''P'' as a function of both ρ and ''T'' (the temperature); however in the particular case described by the polytrope equation there are other additional relations between these three quantities, which together determine the equation. Thus, this is simply a relation that expresses an assumption about the change of pressure with radius in terms of the change of density with radius, yielding a solution to the Lane–Emden equation. Sometimes the word ''polytrope'' may refer to an equation of state that looks similar to the thermodyna ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volume (thermodynamics)
In thermodynamics, the volume of a system is an important extensive parameter for describing its thermodynamic state. The specific volume, an intensive property, is the system's volume per unit of mass. Volume is a function of state and is interdependent with other thermodynamic properties such as pressure and temperature. For example, volume is related to the pressure and temperature of an ideal gas by the ideal gas law. The physical volume of a system may or may not coincide with a control volume used to analyze the system. Overview The volume of a thermodynamic system typically refers to the volume of the working fluid, such as, for example, the fluid within a piston. Changes to this volume may be made through an application of work, or may be used to produce work. An isochoric process however operates at a constant-volume, thus no work can be produced. Many other thermodynamic processes will result in a change in volume. A polytropic process, in particular, causes change ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Process
Classical thermodynamics considers three main kinds of thermodynamic process: (1) changes in a system, (2) cycles in a system, and (3) flow processes. (1)A Thermodynamic process is a process in which the thermodynamic state of a system is changed. A change in a system is defined by a passage from an initial to a final state of thermodynamic equilibrium. In classical thermodynamics, the actual course of the process is not the primary concern, and often is ignored. A state of thermodynamic equilibrium endures unchangingly unless it is interrupted by a thermodynamic operation that initiates a thermodynamic process. The equilibrium states are each respectively fully specified by a suitable set of thermodynamic state variables, that depend only on the current state of the system, not on the path taken by the processes that produce the state. In general, during the actual course of a thermodynamic process, the system may pass through physical states which are not describable as thermody ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adiabatic Process
In thermodynamics, an adiabatic process (Greek: ''adiábatos'', "impassable") is a type of thermodynamic process that occurs without transferring heat or mass between the thermodynamic system and its environment. Unlike an isothermal process, an adiabatic process transfers energy to the surroundings only as work.. A translation may be founhere. Also a mostly reliabltranslation is to be foundin As a key concept in thermodynamics, the adiabatic process supports the theory that explains the first law of thermodynamics. Some chemical and physical processes occur too rapidly for energy to enter or leave the system as heat, allowing a convenient "adiabatic approximation".Bailyn, M. (1994), pp. 52–53. For example, the adiabatic flame temperature uses this approximation to calculate the upper limit of flame temperature by assuming combustion loses no heat to its surroundings. In meteorology and oceanography, adiabatic cooling produces condensation of moisture or salinity, oversatu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
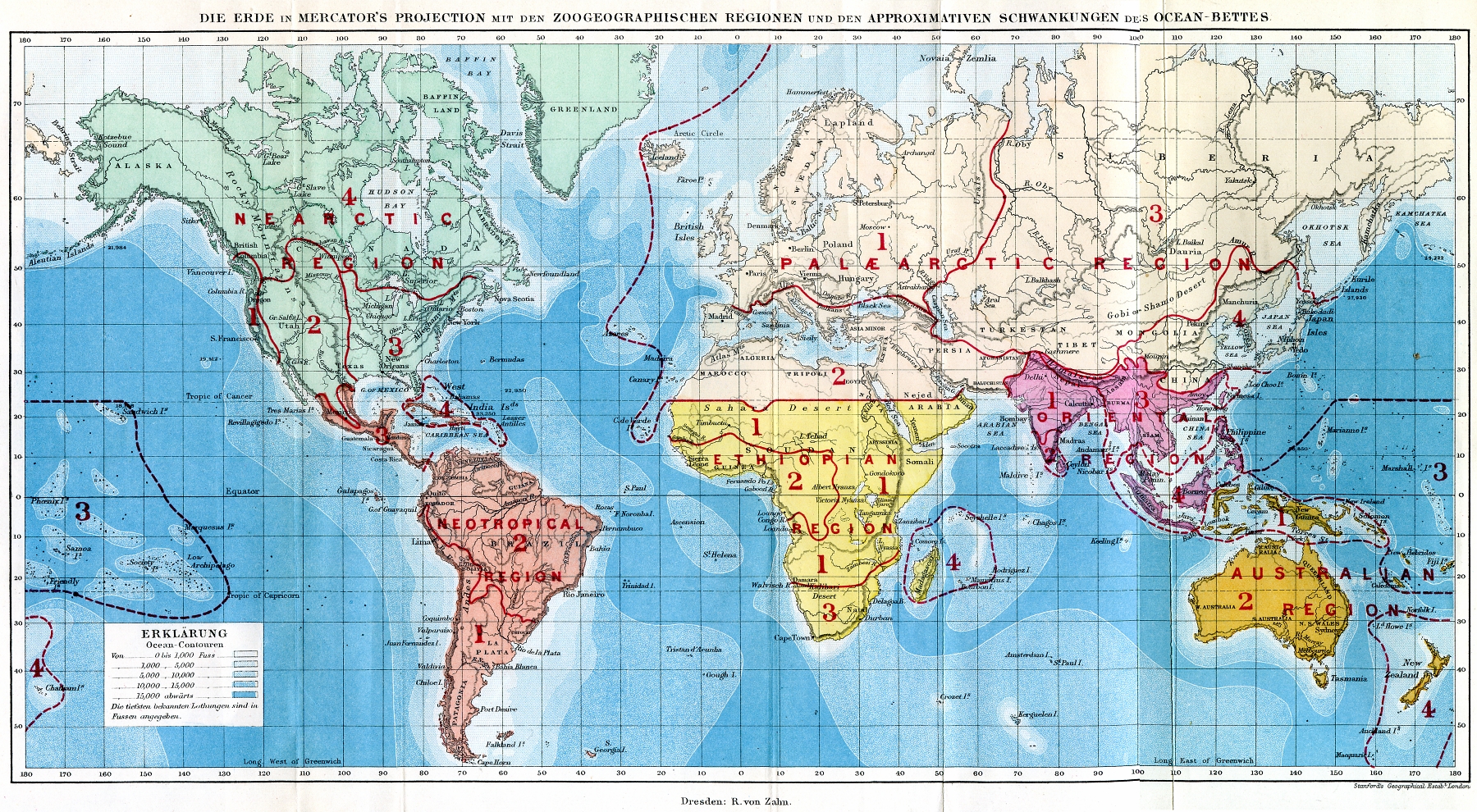
Biogeography
Biogeography is the study of the distribution of species and ecosystems in geographic space and through geological time. Organisms and biological communities often vary in a regular fashion along geographic gradients of latitude, elevation, isolation and habitat area.Brown University, "Biogeography." Accessed February 24, 2014. . Phytogeography is the branch of biogeography that studies the distribution of plants. Zoogeography is the branch that studies distribution of animals. Mycogeography is the branch that studies distribution of fungi, such as mushrooms. Knowledge of spatial variation in the numbers and types of organisms is as vital to us today as it was to our early human ancestors, as we adapt to heterogeneous but geographically predictable environments. Biogeography is an integrative field of inquiry that unites concepts and information from ecology, evolutionary biology, taxonomy, geology, physical geography, palaeontology, and climatology.Dansereau, Pierre. 1957 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isothermal Process
In thermodynamics, an isothermal process is a type of thermodynamic process in which the temperature ''T'' of a system remains constant: Δ''T'' = 0. This typically occurs when a system is in contact with an outside thermal reservoir, and a change in the system occurs slowly enough to allow the system to be continuously adjusted to the temperature of the reservoir through heat exchange (see quasi-equilibrium). In contrast, an ''adiabatic process'' is where a system exchanges no heat with its surroundings (''Q'' = 0). Simply, we can say that in an isothermal process * T = \text * \Delta T = 0 * dT = 0 * For ideal gases only, internal energy \Delta U = 0 while in adiabatic processes: * Q = 0. Etymology The adjective "isothermal" is derived from the Greek words "ἴσος" ("isos") meaning "equal" and "θέρμη" ("therme") meaning "heat". Examples Isothermal processes can occur in any kind of system that has some means of regulating the temperature, including ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isentropic Process
In thermodynamics, an isentropic process is an idealized thermodynamic process that is both adiabatic and reversible. The work transfers of the system are frictionless, and there is no net transfer of heat or matter. Such an idealized process is useful in engineering as a model of and basis of comparison for real processes. This process is idealized because reversible processes do not occur in reality; thinking of a process as both adiabatic and reversible would show that the initial and final entropies are the same, thus, the reason it is called isentropic (entropy does not change). Thermodynamic processes are named based on the effect they would have on the system (ex. isovolumetric: constant volume, isenthalpic: constant enthalpy). Even though in reality it is not necessarily possible to carry out an isentropic process, some may be approximated as such. The word "isentropic" can be interpreted in another way, since its meaning is deducible from its etymology. It means a pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
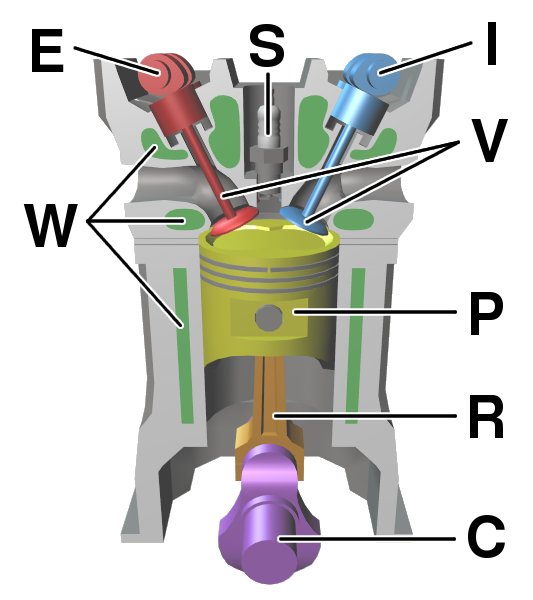
Internal Combustion Engine
An internal combustion engine (ICE or IC engine) is a heat engine in which the combustion of a fuel occurs with an oxidizer (usually air) in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal combustion engine, the expansion of the high-temperature and high-pressure gases produced by combustion applies direct force to some component of the engine. The force is typically applied to pistons ( piston engine), turbine blades (gas turbine), a rotor (Wankel engine), or a nozzle ( jet engine). This force moves the component over a distance, transforming chemical energy into kinetic energy which is used to propel, move or power whatever the engine is attached to. This replaced the external combustion engine for applications where the weight or size of an engine was more important. The first commercially successful internal combustion engine was created by Étienne Lenoir around 1860, and the first modern internal combustion engine, known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mayer's Relation
In the 19th century, German chemist and physicist Julius von Mayer derived a relation between specific heat at constant pressure and the specific heat at constant volume for an ideal gas. Mayer's relation states that :C_ - C_ = R, where is the molar specific heat at constant pressure, is the molar specific heat at constant volume and is the gas constant. For more general homogeneous substances, not just ideal gases, the difference takes the form, :C_ - C_= V_ T\frac\, (see relations between heat capacities), where V_ is the molar volume, T is the temperature, \alpha_ is the thermal expansion coefficient and \beta is the isothermal compressibility. From this latter relation, several inferences can be made: * Since the isothermal compressibility \beta_ is positive for nearly all phases, and the square of thermal expansion coefficient is always either a positive quantity or zero, the specific heat at constant pressure is nearly always greater than or equal to specific heat at c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |