|
Polydimethylsiloxane
Polydimethylsiloxane (PDMS), also known as dimethylpolysiloxane or dimethicone, belongs to a group of polymeric organosilicon compounds that are commonly referred to as silicones. PDMS is the most widely used silicon-based organic polymer, as its versatility and properties lead to many applications. It is particularly known for its unusual rheological (or flow) properties. PDMS is optically clear and, in general, inert, non-toxic, and non-flammable. It is one of several types of silicone oil (polymerized siloxane). Its applications range from contact lenses and medical devices to elastomers; it is also present in shampoos (as it makes hair shiny and slippery), food (antifoaming agent), caulk, lubricants and heat-resistant tiles. Structure The chemical formula of PDMS is , where ''n'' is the number of repeating monomer units.Mark, J. E.; Allcock, H. R.; West, R. “Inorganic Polymers” Prentice Hall, Englewood, NJ: 1992. . Industrial synthesis can begin from dimethyldichloro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicone
A silicone or polysiloxane is a polymer made up of siloxane (−R2Si−O−SiR2−, where R = organic group). They are typically colorless oils or rubber-like substances. Silicones are used in sealants, adhesives, lubricants, medicine, cooking utensils, thermal insulation, and electrical insulation. Some common forms include silicone oil, silicone grease, silicone rubber, silicone resin, and silicone caulk. Chemistry More precisely called polymerized siloxanes or polysiloxanes, silicones consist of an inorganic silicon–oxygen backbone chain (⋯−Si−O−Si−O−Si−O−⋯) with two organic groups attached to each silicon center. Commonly, the organic groups are methyl. The materials can be cyclic or polymeric. By varying the −Si−O− chain lengths, side groups, and crosslinking, silicones can be synthesized with a wide variety of properties and compositions. They can vary in consistency from liquid to gel to rubber to hard plastic. The most common siloxan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antifoaming Agent
A defoamer or an anti-foaming agent is a chemical additive that reduces and hinders the formation of foam in industrial process liquids. The terms anti-foam agent and defoamer are often used interchangeably. Strictly speaking, defoamers eliminate existing foam and anti-foamers prevent the formation of further foam. Commonly used agents are insoluble oils, polydimethylsiloxanes and other silicones, certain alcohols, stearates and glycols. The additive is used to prevent formation of foam or is added to break a foam already formed. In industrial processes, foams pose serious problems. They cause defects on surface coatings and prevent the efficient filling of containers. A variety of chemical formulae are available to prevent formation of foams. Properties Generally a defoamer is insoluble in the foaming medium and has surface active properties. An essential feature of a defoamer product is a low viscosity and a facility to spread rapidly on foamy surfaces. It has affinity to t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosilicon
Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an ''inorganic'' compound. History In 1846 Von Ebelman's had synthesized Tetraethyl orthosilicate (Si(OC2H5)4). In 1863 Friedel and Crafts managed to make the first organosilieon compound with C-Si bonds which gone byound the syntheses of orthosilicic acid esters. The same year they also described a «polysilicic acid ether» in the preparation of ethyl- and methyl-o-silicic acid. The early extensive research in the field of organosilicon compounds was pioneerd in the beginning of 20th century by Frederic Kipping. He also had coined the term «silicone» (akin to ketones) in relation to these materials in 1904. In recognition of Kipping's achiev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Siloxane
A siloxane is a functional group in organosilicon chemistry with the Si−O−Si linkage. The parent siloxanes include the oligomeric and polymeric hydrides with the formulae H(OSiH2)''n''OH and (OSiH2)n. Siloxanes also include branched compounds, the defining feature of which is that each pair of silicon centres is separated by one oxygen (O2-) atom. The siloxane functional group forms the backbone of silicones, the premier example of which is polydimethylsiloxane (PDMS). The functional group R3SiO− (where the three Rs may be different) is called siloxy. Siloxanes are manmade and have many commercial and industrial applications because of the compounds’ hydrophobicity, low thermal conductivity, and high flexibility. Structure Siloxanes generally adopt structures expected for linked tetrahedral ("''sp''3-like") centers. The Si−O bond length is 1.64 Å (vs Si–C distance of 1.92 Å) and the Si-O-Si angle is rather open at 142.5°. By contrast, the C−O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tiles
Tiles are usually thin, square or rectangular coverings manufactured from hard-wearing material such as ceramic, stone, metal, baked clay, or even glass. They are generally fixed in place in an array to cover roofs, floors, walls, edges, or other objects such as tabletops. Alternatively, tile can sometimes refer to similar units made from lightweight materials such as perlite, wood, and mineral wool, typically used for wall and ceiling applications. In another sense, a tile is a construction tile or similar object, such as rectangular counters used in playing games (see tile-based game). The word is derived from the French word ''tuile'', which is, in turn, from the Latin word ''tegula'', meaning a roof tile composed of fired clay. Tiles are often used to form wall and floor coverings, and can range from simple square tiles to complex or mosaics. Tiles are most often made of ceramic, typically glazed for internal uses and unglazed for roofing, but other materials are also c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caulk
Caulk or, less frequently, caulking is a material used to seal joints or seams against leakage in various structures and piping. The oldest form of caulk consisted of fibrous materials driven into the wedge-shaped seams between boards on wooden boats or ships. Cast iron sewerage pipe were formerly caulked in a similar way. Riveted seams in ships and boilers were formerly sealed by hammering the metal. Modern caulking compounds are flexible sealing compounds used to close up gaps in buildings and other structures against water, air, dust, insects, or as a component in firestopping. In the tunnelling industry, caulking is the sealing of joints in segmental precast concrete tunnels, commonly by using concrete. Historical uses Wooden shipbuilding Traditional caulking (also spelled calking) on wooden vessels uses fibers of cotton and oakum (hemp) soaked in pine tar. These fibers are driven into the wedge-shaped seam between planks, with a caulking mallet and a broad chisel-lik ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lubricant
A lubricant (sometimes shortened to lube) is a substance that helps to reduce friction between surfaces in mutual contact, which ultimately reduces the heat generated when the surfaces move. It may also have the function of transmitting forces, transporting foreign particles, or heating or cooling the surfaces. The property of reducing friction is known as lubricity. In addition to industrial applications, lubricants are used for many other purposes. Other uses include cooking (oils and fats in use in frying pans, in baking to prevent food sticking), bioapplications on humans (e.g. lubricants for artificial joints), ultrasound examination, medical examination, and sexual intercourse. It is mainly used to reduce friction and to contribute to a better and efficient functioning of a mechanism. History Lubricants have been in some use for thousands of years. Calcium soaps have been identified on the axles of chariots dated to 1400 BC. Building stones were slid on oil-impregrated lumb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Resistance
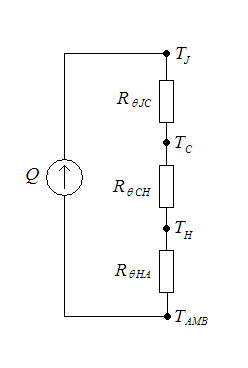
Thermal resistance is a heat property and a measurement of a temperature difference by which an object or material resists a heat flow. Thermal resistance is the reciprocal of thermal conductance. * (Absolute) thermal resistance ''R'' in kelvins per watt (K/W) is a property of a particular component. For example, a characteristic of a heat sink. * Specific thermal resistance or thermal resistivity ''Rλ'' in kelvin–metres per watt (K⋅m/W), is a material constant. * Thermal insulance has the units square metre kelvin per watt (m2⋅K/W) in SI units or square foot degree Fahrenheit– hours per British thermal unit (ft2⋅°F⋅h/Btu) in imperial units. It is the thermal resistance of unit area of a material. In terms of insulation, it is measured by the R-value. Absolute thermal resistance Absolute thermal resistance is the temperature difference across a structure when a unit of heat energy flows through it in unit time. It is the reciprocal of thermal conductance. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass Transition
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased. ISO 11357-2: Plastics – Differential scanning calorimetry – Part 2: Determination of glass transition temperature (1999). An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification. The glass-transition temperature ''T''g of a material characterizes the range of temperatures over which this glass transition occurs (as an experimental definition, typically marked as 100 s of relaxation time). It is always lower than the melting temperature, ''T''m, of the crystalline state of the material, if one exists. Hard plastics like polystyrene and poly(methyl methacrylate) are u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include Subscript and superscript, subscripts and superscripts. A chemical formula is not a chemical nomenclature, chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called ''empirical formulae'', which use letters and numbers ind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Slippery
Slipperiness is when a surface has a low coefficient of friction, allowing objects to glide across the surface. People walking on slippery surfaces are likely to slip or fall. A surface can for example be slippery due to it being wet, or due to it being icy. There are several competing theories about why ice is slippery. Road slipperiness is a major area of road safety, but various means have also been developed to measure walkway and deck slipperiness in order to develop health and safety standards.Wen-Ruey Chang, Theodore K. Courtney, Raoul Grongvist ''Measuring Slipperiness: Human Locomotion and Surface Factors'' 2002 0415298288 "A number of 'purely' subjective approaches (e.g. paired comparisons) have been applied to measure slipperiness. Human subjects seem to be capable of differentiating the slipperiness of floors (Yoshioka et al. 1978, 1979, Swensen et al." See also *Floor slip resistance testing *Ice cleats Ice cleats are a contraption, affixed to a shoe or boot, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyldichlorosilane
Dimethyldichlorosilane is a tetrahedral, organosilicon compound with the formula Si(CH3)2Cl2. At room temperature it is a colorless liquid that readily reacts with water to form both linear and cyclic Si-O chains. Dimethyldichlorosilane is made on an industrial scale as the principal precursor to dimethylsilicone and polysilane compounds. History The first organosilicon compounds were reported in 1863 by Charles Friedel and James Crafts who synthesized tetraethylsilane from diethylzinc and silicon tetrachloride.Silicon: Organosilicon Chemistry. Encyclopedia of Inorganic Chemistry Online, 2nd ed.; Wiley: New Jersey, 2005. However, major progress in organosilicon chemistry did not occur until Frederick Kipping and his students began experimenting with diorganodichlorosilanes (R2SiCl2) that were prepared by reacting silicon tetrachloride with Grignard reagents. Unfortunately, this method suffered from many experimental problems. In the 1930s, the demand for silicones increased ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |






_in_1890_09.jpg)
_【_Pictures_taken_in_Japan_】_(cropped).jpg)