Siloxane on:
[Wikipedia]
[Google]
[Amazon]
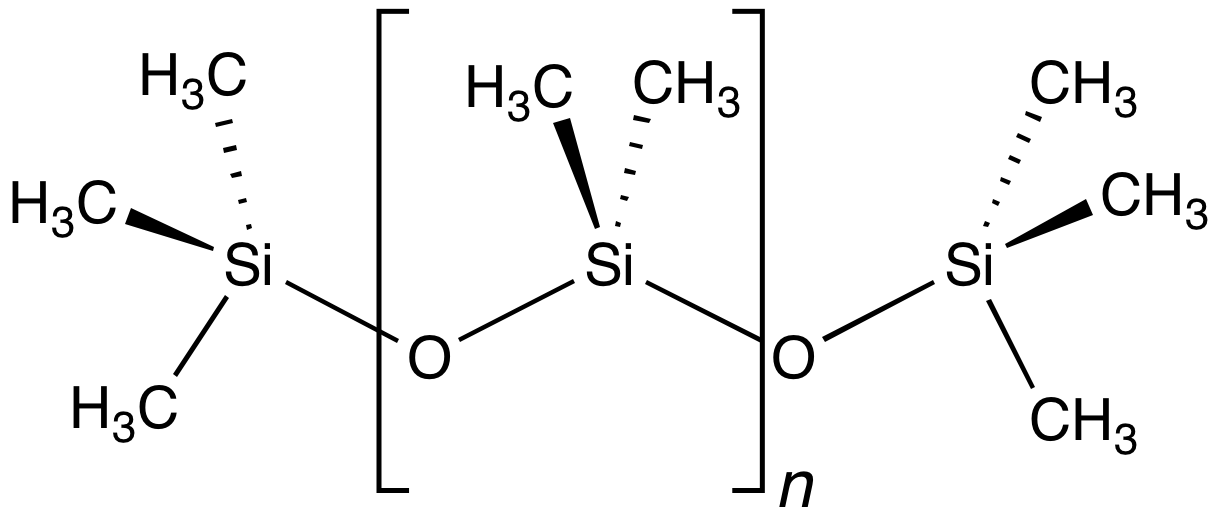 A siloxane is a functional group in
A siloxane is a functional group in
 The word ''siloxane'' is derived from the words silicon, oxygen, and alkane. In some cases, siloxane materials are composed of several different types of siloxane groups; these are labeled according to the number of Si−O bonds:
: M-units: (CH3)3SiO0.5,
: D-units: (CH3)2SiO,
: T-units: (CH3)SiO1.5.
The word ''siloxane'' is derived from the words silicon, oxygen, and alkane. In some cases, siloxane materials are composed of several different types of siloxane groups; these are labeled according to the number of Si−O bonds:
: M-units: (CH3)3SiO0.5,
: D-units: (CH3)2SiO,
: T-units: (CH3)SiO1.5.
Report of the Board of Review for Decamethylcyclopentasiloxane (Siloxane D5) established under Section 333(1) of the Canadian Environmental Protection Act of 1999, October 20, 2011
/ref>
EPA report: Siloxane D5 in Dry-cleaning
Journal Of Protective Coatings And Linings: Field Performance of Polysiloxanes
*
 A siloxane is a functional group in
A siloxane is a functional group in organosilicon
Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic c ...
chemistry with the Si−O−Si linkage. The parent siloxanes include the oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relati ...
ic and polymer
A polymer (; Greek '' poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
ic hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
s with the formulae H(OSiH2)''n''OH and (OSiH2)n. Siloxanes also include branched compounds, the defining feature of which is that each pair of silicon centres is separated by one oxygen (O2-) atom. The siloxane functional group forms the backbone of silicone
A silicone or polysiloxane is a polymer made up of siloxane (−R2Si−O−SiR2−, where R = organic group). They are typically colorless oils or rubber-like substances. Silicones are used in sealants, adhesives, lubricants, medicine, cookin ...
s, the premier example of which is polydimethylsiloxane
Polydimethylsiloxane (PDMS), also known as dimethylpolysiloxane or dimethicone, belongs to a group of polymeric organosilicon compounds that are commonly referred to as silicones. PDMS is the most widely used silicon-based organic polymer, as its ...
(PDMS). The functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the r ...
R3SiO− (where the three Rs may be different) is called siloxy. Siloxanes are manmade and have many commercial and industrial applications because of the compounds’ hydrophobicity, low thermal conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa.
Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal ...
, and high flexibility.
Structure
Siloxanes generally adopt structures expected for linked tetrahedral ("''sp''3-like") centers. The Si−O bond length is 1.64 Å (vs Si–C distance of 1.92 Å) and the Si-O-Si angle is rather open at 142.5°. By contrast, the C−O distance in a typical dialkyl ether is much shorter at 1.414(2) Å with a more acute C−O−C angle of 111°. It can be appreciated that the siloxanes would have low barriers for rotation about the Si−O bonds as a consequence of low steric hindrance. This geometric consideration is the basis of the useful properties of some siloxane-containing materials, such as their lowglass transition temperature
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or ru ...
s.
Synthesis of siloxanes
140 px, Dimethyldichlorosilane (Si(CH3)2Cl2) is a key precursor to cyclic (D3, D4, etc.) and linear siloxanes.Silicon: Organosilicon Chemistry. Encyclopedia of Inorganic Chemistry Online, 2nd ed.; Wiley: New Jersey, 2005. . The main route to siloxane functional group is by hydrolysis of silicon chloride, silicon chlorides: : 2 R3Si−Cl + H2O → R3Si−O−SiR3 + 2 HCl The reaction proceeds via the initial formation of silanols (R3Si−OH): : R3Si−Cl + H2O → R3Si−OH + HCl The siloxane bond can then form via a silanol + silanol pathway or a silanol + chlorosilane pathway: : 2 R3Si−OH → R3Si−O−SiR3 + H2O : R3Si−OH + R3Si−Cl → R3Si−O−SiR3 + HCl Hydrolysis of a silyldichloride can afford linear or cyclic products. Linear products are terminated with silanol groups: : ''n'' R2Si(OH)2 → H(R2SiO)''n''OH + (''n'' − 1) H2O Cyclic products have no silanol termini: : ''n'' R2Si(OH)2 → (R2SiO)''n'' + ''n'' H2O The linear products,polydimethylsiloxane
Polydimethylsiloxane (PDMS), also known as dimethylpolysiloxane or dimethicone, belongs to a group of polymeric organosilicon compounds that are commonly referred to as silicones. PDMS is the most widely used silicon-based organic polymer, as its ...
(PDMS), are of great commercial value. Their production requires the production of dimethylsilicon dichloride
Dimethyldichlorosilane is a tetrahedral, organosilicon compound with the formula Si(CH3)2Cl2. At room temperature it is a colorless liquid that readily reacts with water to form both linear and cyclic Si-O chains. Dimethyldichlorosilane is made ...
.
Starting from trisilanols, cages are possible, such as the species with the formula (RSi)''n''O3''n''/2 with cubic (''n'' = 8) and hexagonal prismatic (''n'' = 12) structures. The cubic cages are cubane-type cluster
A cubane-type cluster is an arrangement of atoms in a molecular structure that forms a cube. In the idealized case, the eight vertices are symmetry equivalent and the species has Oh symmetry. Such a structure is illustrated by the hydrocarbon ...
s, with silicon centers at the corners of a cube oxygen centres spanning each of the twelve edges.
Reactions
Oxidation of organosilicon compounds, including siloxanes, givessilicon dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one ...
. This conversion is illustrated by the combustion of hexamethylcyclotrisiloxane:
:((CH3)2SiO)3 + 12 O2 → 3 SiO2 + 6 CO2 + 9 H2O
Strong base degrades siloxane group, often affording siloxide salts
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively c ...
:
:((CH3)3Si)2O + 2 NaOH → 2 (CH3)3SiONa + H2O
This reaction proceeds by production of silanols. Similar reactions are used industrially to convert cyclic siloxanes to linear polymers.Röshe, L.; John, P.; Reitmeier, R. "Organic Silicon Compounds" Ullmann’s Encyclopedia of Industrial Chemistry. John Wiley and Sons: San Francisco, 2003. .
Uses
Polysiloxanes (silicones), upon combustion in an inert atmosphere, generally undergo pyrolysis to form silicon oxycarbide orsilicon carbide
Silicon carbide (SiC), also known as carborundum (), is a hard chemical compound containing silicon and carbon. A semiconductor, it occurs in nature as the extremely rare mineral moissanite, but has been mass-produced as a powder and crystal s ...
(SiC). By exploiting this reaction, polysiloxanes have been used as preceramic polymers in various processes including additive manufacturing. Polyvinyl siloxane (vinyl polysiloxane) is used to make dental impressions and industrial impressions. The use of a poly-siloxane precursor in polymer derived ceramics allows the formation of ceramic bodies with complex shapes, although the significant shrinkage in pyrolysis needs to be taken into account.
Cyclomethicones
Cyclomethicones are a group of methyl siloxanes, a class of liquid silicones (cyclic polydimethylsiloxane polymers) that possess the characteristics of lowviscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the int ...
and high volatility as well as being skin emollient
A moisturizer, or emollient, is a cosmetic preparation used for protecting, moisturizing, and lubricating the skin. These functions are normally performed by sebum produced by healthy skin. The word "emollient" is derived from the Latin verb ''m ...
s and in certain circumstances useful cleaning solvents. Unlike dimethicones, which are ''linear'' siloxanes that do not evaporate
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. High concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humi ...
, cyclomethicones are ''cyclic'': both groups consist of a backbone of CH3)2SiOsub>n. They are used in many cosmetic products including deodorants and antiperspirants which need to coat the skin but not remain tacky afterward. Dow is a major producer of cyclomethicones.
Cyclomethicones, like all siloxanes, degrade by hydrolysis, producing silanols. These silanols are produced at such low levels that they do not interfere with hydrolytic enzymes. Even though some cyclomethicones structurally resemble crown ethers, they bind metal ions only weakly.
Nomenclature
Safety and environmental considerations
Because silicones are heavily used in biomedical and cosmetic applications, their toxicology has been intensively examined. "The inertness of silicones toward warmblooded animals has been demonstrated in a number of tests." With an LD50 in rats of >50 g/kg, they are virtually nontoxic. Questions remain however about chronic toxicity or the consequences of bioaccumulation since siloxanes can be long-lived. Findings about bioaccumulation have been largely based on laboratory studies. Field studies of bioaccumulation have not reached consensus. "Even if the concentrations of siloxanes we have found in fish are high compared to concentrations of classical contaminants like PCBs, several other studies in the Oslo Fjord in Norway, Lake Pepin in the US, andLake Erie
Lake Erie ( "eerie") is the fourth largest lake by surface area of the five Great Lakes in North America and the eleventh-largest globally. It is the southernmost, shallowest, and smallest by volume of the Great Lakes and therefore also ha ...
in Canada have shown concentrations of siloxanes decrease at higher range in the food chain. This finding raises questions about which factors influence the bioaccumulation potential of siloxanes."
Cyclomethicones are ubiquitous because they are widely used in biomedical and cosmetic applications. They can be found at high levels in American cities. They can be toxic to aquatic animals in concentrations often found in the environment. The cyclomethicones D4 and D5 are bioaccumulative
Bioaccumulation is the gradual accumulation of substances, such as pesticides or other chemicals, in an organism. Bioaccumulation occurs when an organism absorbs a substance at a rate faster than that at which the substance is lost or eliminated ...
in some aquatic organisms, according to one report.
In the European Union, D4, D5 and D6 have been deemed hazardous as per the REACH
Reach or REACH may refer to:
Companies and organizations
* Reach plc, formerly Trinity Mirror, large British newspaper, magazine, and digital publisher
* Reach Canada, an NGO in Canada
* Reach Limited, an Asia Pacific cable network company ...
regulation. They were characterized as substances of very high concern (SVHC) due to their PBT and vPvB properties. Canada regulates D4 under a pollution prevention plan. A scientific review in Canada in 2011 concluded that "Siloxane D5 does not pose a danger to the environment."/ref>
Literature
* Christoph Rücker, Klaus Kümmerer: ''Environmental Chemistry of Organosiloxanes.'' In: ''Chemical Reviews
''Chemical Reviews'' is peer-reviewed scientific journal published twice per month by the American Chemical Society. It publishes review articles on all aspects of chemistry. It was established in 1924 by William Albert Noyes ( University of Il ...
.'' 115(1), 2015, p. 466–524, .
References
{{reflistExternal links
EPA report: Siloxane D5 in Dry-cleaning
Journal Of Protective Coatings And Linings: Field Performance of Polysiloxanes
*