|
Polyamines
A polyamine is an organic compound having more than two amino groups. Alkyl polyamines occur naturally, but some are synthetic. Alkylpolyamines are colorless, hygroscopic, and water soluble. Near neutral pH, they exist as the ammonium derivatives. Most aromatic polyamines are crystalline solids at room temperature. Natural polyamines Low-molecular-weight linear polyamines are found in all forms of life. The principal examples are the triamine spermidine and the tetraamine spermine. They are structurally and biosynthetically related to the diamines putrescine and cadaverine. Polyamine metabolism is regulated by the activity of the enzyme ornithine decarboxylase (ODC). Polyamines are found in high concentrations in the mammalian brain. File:Spermidine-2D-skeletal.svg, spermidine File:Spermine.svg, spermine Synthetic polyamines Several synthetic polyamines are used in chemical industry and the research laboratory. They are mainly of interest as additives to motor oil and as co-rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spermidine
Spermidine is a polyamine compound () found in ribosomes and living tissues and having various metabolic functions within organisms. It was originally isolated from semen. Function Spermidine is an aliphatic polyamine. Spermidine synthase (SPDS) catalyzes its formation from putrescine. It is a precursor to other polyamines, such as spermine and its structural isomer thermospermine. Spermidine synchronizes an array of biological processes, (such as Ca2+, Na+, K+ -ATPase) thus maintaining membrane potential and controlling intracellular pH and volume. Spermidine regulates biological processes, such as Ca2+ influx by glutamatergic N-methyl-d-aspartate receptor (NMDA receptor), which has been associated with nitric oxide synthase (NOS) and cGMP/PKG pathway activation and a decrease of Na+,K+-ATPase activity in cerebral cortex synaptosomes. Spermidine is a longevity agent in mammals due to various mechanisms of action, which are just beginning to be understood. Autophagy is the main m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ornithine Decarboxylase
The enzyme ornithine decarboxylase (, ODC) catalyzes the decarboxylation of ornithine (a product of the urea cycle) to form putrescine. This reaction is the committed step in polyamine synthesis. In humans, this protein has 461 amino acids and forms a homodimer. Reaction mechanism Lysine 69 on ornithine decarboxylase (ODC) binds the cofactor pyridoxal phosphate to form a Schiff base. Ornithine displaces the lysine to form a Schiff base attached to orthonine, which decarboxylates to form a quinoid intermediate. This intermediate rearranges to form a Schiff base attached to putrescine, which is attacked by lysine to release putrescine product and reform PLP-bound ODC. This is the first step and the rate-limiting step in humans for the production of polyamines, compounds required for cell division. Structure image:Ornithine Decarboxylase Publication View.png, 270px, 3D crystal structure of ornithine decarboxylase.; ; rendered viPyMOL The active form of ornithine decarboxyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spermine
Spermine is a polyamine involved in cellular metabolism that is found in all Eukaryote, eukaryotic cells. The precursor for synthesis of spermine is the amino acid ornithine. It is an essential growth factor in some Bacterium, bacteria as well. It is found as a polycation at physiological pH. Spermine is associated with nucleic acids and is thought to stabilize helical structure, particularly in viruses. Antonie van Leeuwenhoek first described crystals of spermine phosphate in human semen in 1678. The name ''spermin'' was first used by the German chemists Albert Ladenburg, Ladenburg and Abel in 1888, and the correct structure of spermine was not finally established until 1926, simultaneously in England (by Dudley, Rosenheim, and Starling) and Germany (by Wrede et al.). Spermine is the chemical primarily responsible for the characteristic odor of semen. Derivative A derivative (chemistry), derivative of spermine, N1, N12-bis(ethyl)spermine (also known as BESm) was investig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
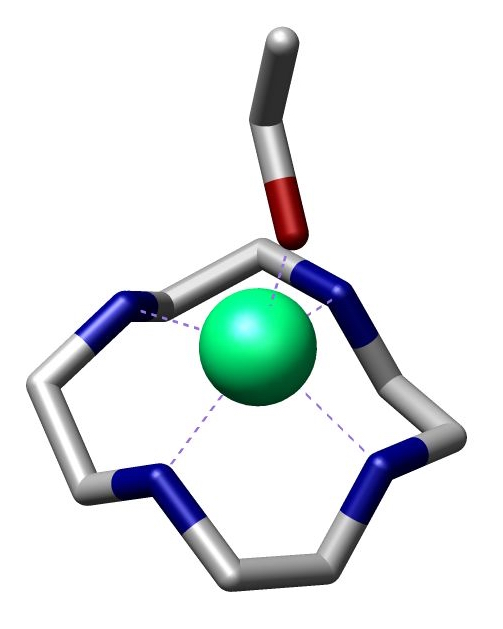
Cyclam
Cyclam (1,4,8,11-tetraazacyclotetradecane) is an organic compound with the formula (NHCH2CH2NHCH2CH2CH2)2. Classified as an aza-crown ether, it is a white solid that is soluble in water. As a macrocyclic ligand, it binds strongly to many transition metal cations. The compound was first prepared by the reaction of 1,3-dibromopropane and ethylenediamine. The compound features four secondary amines. Its complexes therefore can exist as several diastereomers, depending on the relative orientation of the N–H centres. Its complexes feature alternating five- and six-membered chelate rings. The closely related ligand cyclen ((CH2CH2NH)4) forms only five-membered C2N2M chelate rings and tends not to form square-planar complexes. ''N''-Alkyl derivatives Metal-cyclam complexes are prone to oxidative degradation, which is initiated by deprotonation of the secondary amine. This flaw led to the development of cyclam derivatives wherein the NH centres are replaced by tertiary amines. For ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1,1-Tris(aminomethyl)ethane
1,1,1-Tris(aminomethyl)ethane (TAME) is an organic compound with the formula CHC(CHNH). It is a colorless liquid. It is classified as a polyamine tripodal ligand, i.e., capable of binding to metal ions through three sites and hence is a tridentate chelating ligand, occupying a face of the coordination polyhedron. Preparation TAME is synthesized by the Pd/C-catalyzed hydrogenation of 1,1,1-tris(azidomethyl)ethane. Although azides are potentially explosive, they are excellent and practical source of primary amines. The required tris(azidomethyl)ethane is obtained from the tri tosylate by salt metathesis using sodium azide. These two steps are: :3 NaN + CHC(CHOTs) → CHC(CHN) + 3 NaOTs :3 H + CHC(CHN) → CHC(CHNH) + 3 N Complexes of TAME The tripodal TAME ligand coordinates facially to metal ions. This stereochemical feature has been exploited in the preparation of platinum(IV) cage complexes, e.g., t(tame) which is a six coordinate Pt(IV) complex. Platinu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triazinane
Triazinanes are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is . They exist in three isomeric forms, 1,3,5-triazinanes being common. The triazinanes have six-membered cyclohexane-like ring but with three carbons replaced by nitrogens. Most commonly, the amines are tertiary. References * ''Heterocyclic Chemistry'' T.L. Gilchrist 1985 (1997, ) See also * 6-membered rings with one nitrogen atom: Piperidine * 6-membered rings with two nitrogen atoms: Diazinane ** Hexahydropyrimidine ** Hexahydropyridazine * Triazine Triazines are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is . They exist in three isomeric forms, 1,3,5-triazines being common. Structure The triazines have planar six-membered benzene-like ring but ... {{Authority control Heterocyclic compounds with 1 ring Nitrogen heterocycles Six-membered rings Polyamines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,4,7-Triazacyclononane
1,4,7-Triazacyclononane, known as "TACN" which is pronounced "tack-en," is an aza-crown ether with the formula (C2H4NH)3. TACN is derived, formally speaking, from cyclononane by replacing three equidistant CH2 groups with NH groups. TACN is one of the oligomers derived from aziridine, C2H4NH. Other members of the series include piperazine, C4H8(NH)2, and the cyclic tetramer 1,4,7,10-tetraazacyclododecane. Synthesis The ligand is prepared from diethylene triamine as follows by macrocyclization using ethyleneglycol ditosylate. :H2NCH2CH2NHCH2CH2NH2 + 3 TsCl → Ts(H)NCH2CH2N(Ts)CH2CHH2N(H)Ts + 3 HCl :Ts(H)NCH2CH2N(Ts)CH2CH2N(H)Ts + 2 NaOEt → Ts(Na)NCH2CH2N(Ts)CH2CH2N(Na)Ts :Ts(Na)NCHH2CH2N(Ts)CH2CH2N(Na)Ts + TsOCH2CH2OTs + → CH2CH2N(Ts)sub>3 + 2 NaOTs : CH2CH2N(Ts)sub>3 + 3 H2O → H2CH2NHsub>3 + 3 HOTs Coordination chemistry TACN is a popular tridentate ligand. It is threefold symmetric and binds to one face of an octahedron of metalloids and transition ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclen
Cyclen (1,4,7,10-tetraazacyclododecane) is a aza-crown ether with the formula (CH2CH2NH)4. It is a white solid. Synthesis Some syntheses exploit the Thorpe-Ingold effect to facilitate ring-formation. Illustrative is the reaction of the deprotonated tosylamides with ditosylates: :TsN(CH2CH2NTsNa)2 + TsN(CH2CH2OTs)2 → (TsNCH2CH2)4 The resulting macrocycle can be deprotected with strong acid. Base gives the tetramine. High dilution conditions result in a low reaction rate penalty and this disadvantage is removed in an alternative procedure starting from triethylenetetraamine and dithiooxamide to a bisamidine – also a bis(imidazoline) – followed by reduction and ring expansion with DIBAL. : In one study cyclen is covalently bonded through a propylene molecular spacer to adenine and chelated with zinc diperchlorate. This complex is able to selectively bind uracil and uridine in a 1:2 ratio both through the adenine part and cyclen part of the molecule as evi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tris(2-aminoethyl)amine
Tris(2-aminoethyl)amine is the organic compound with the chemical formula, formula N(CH2CH2NH2)3. This colourless liquid is soluble in water and is highly basic, consisting of a tertiary amine center and three pendant primary amine groups. Abbreviated tren or TREN it is a crosslinking agent in the synthesis of polyimine networks and a tripodal ligand in coordination chemistry. Tren is a C3-symmetric, tetradentate chelating ligand that forms stable complexes with transition metals, especially those in the 2+ and 3+ oxidation states. Tren complexes exist with relatively few isomers, reflecting the constrained connectivity of this tetramine. Thus, only a single achiral stereoisomer exists for [Co(tren)X2]+, where X is halide or pseudohalide. In contrast, for [Co(trien)X2]+ five diastereomers are possible, four of which are chiral. In a few cases, tren serves as a tridentate ligand with one of the primary amine groups non-coordinated. Tren is a common impurity in the more common trie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyethylenimine
Polyethylenimine (PEI) or polyaziridine is a polymer with repeating units composed of the amine group and two carbon aliphatic ''CHCH'' spacers. Linear polyethyleneimines contain all secondary amines, in contrast to branched PEIs which contain primary, secondary and tertiary amino groups. Totally branched, dendrimeric forms were also reported. PEI is produced on an industrial scale and finds many applications usually derived from its polycationic character. Properties The linear PEI is a semi-crystalline solid at room temperature while branched PEI is a fully amorphous polymer existing as a liquid at all molecular weights. Linear polyethyleneimine is soluble in hot water, at low pH, in methanol, ethanol, or chloroform. It is insoluble in cold water, benzene, ethyl ether, and acetone. Linear polyethyleneimine has a melting point of around 67 °C. Both linear and branched polyethyleneimine can be stored at room temperature. Linear polyethyleneimine is able to form cryogels up ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cl2.png)