|
Photooxygenation
A photooxygenation is a light-induced oxidation reaction in which molecular oxygen is incorporated into the product(s). Initial research interest in photooxygenation reactions arose from Oscar Raab's observations in 1900 that the combination of light, oxygen and photosensitizers is highly toxic to cells. Early studies of photooxygenation focused on oxidative damage to DNA and amino acids, but recent research has led to the application of photooxygenation in organic synthesis and photodynamic therapy. Photooxygenation reactions are initiated by a photosensitizer, which is a molecule that enters an excited state when exposed to light of a specific wavelength (e.g. dyes and pigments). The excited sensitizer then reacts with either a substrate or ground state molecular oxygen, starting a cascade of energy transfers that ultimately result in an oxygenated molecule. Consequently, photooxygenation reactions are categorized by the type and order of these intermediates (as type I, type II, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photooxygenation Venn Diagram
A photooxygenation is a light-induced oxidation reaction in which molecular oxygen is incorporated into the product(s). Initial research interest in photooxygenation reactions arose from Oscar Raab's observations in 1900 that the combination of light, oxygen and photosensitizers is highly toxic to cells. Early studies of photooxygenation focused on oxidative damage to DNA and amino acids, but recent research has led to the application of photooxygenation in organic synthesis and photodynamic therapy. Photooxygenation reactions are initiated by a photosensitizer, which is a molecule that enters an excited state when exposed to light of a specific wavelength (e.g. dyes and pigments). The excited sensitizer then reacts with either a substrate or ground state molecular oxygen, starting a cascade of energy transfers that ultimately result in an oxygenated molecule. Consequently, photooxygenation reactions are categorized by the type and order of these intermediates (as type I, type II, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photooxygenation Type Diagram
A photooxygenation is a light-induced oxidation reaction in which molecular oxygen is incorporated into the product(s). Initial research interest in photooxygenation reactions arose from Oscar Raab's observations in 1900 that the combination of light, oxygen and photosensitizers is highly toxic to cells. Early studies of photooxygenation focused on oxidative damage to DNA and amino acids, but recent research has led to the application of photooxygenation in organic synthesis and photodynamic therapy. Photooxygenation reactions are initiated by a photosensitizer, which is a molecule that enters an excited state when exposed to light of a specific wavelength (e.g. dyes and pigments). The excited sensitizer then reacts with either a substrate or ground state molecular oxygen, starting a cascade of energy transfers that ultimately result in an oxygenated molecule. Consequently, photooxygenation reactions are categorized by the type and order of these intermediates (as type I, type II, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schenck Ene Reaction
Jewish (Ashkenazic) and German occupational surname derived from ''schenken'' (to pour out or serve) referring to the medieval profession of cup-bearer or wine server (later also to tavern keeper). At one time only Jews were allowed to sell alcohol in the Russian empire, which is why Shenk (Russian) and its later surname variants are very common.http://www.ancestry.com/name-origin?te=5&surname=schenk People with this surname include: People * Adolph Schenck (1803–1878), German teacher and entomologist * Aubrey Schenck (1908–1999), film producer * August Friedrich Schenck (1828–1901), German painter * Carl Alwyn Schenck (1868–1955), German pioneer of forestry in the USA and Europe * Carl Schenck (1835–1910), German mercantilist and founder of the Carl Schenck Eisengießerei & Waagenfabrik * Charles Schenck, American socialist * Ernst-Günther Schenck (1904–1998), German doctor * Ferdinand Schureman Schenck (1790–1860), American physician and politician * Fred ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Structure
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the chemical bonding, bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as complex (chemistry), coordination compounds. The Lewis structure was named after Gilbert N. Lewis, who introduced it in his 1916 article ''The Atom and the Molecule.'' Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another (pairs of dots can be used instead of lines). Excess electrons that form lone pairs are represented as pairs of dots, and are placed next to the at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloadditions
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". The resulting reaction is a cyclization reaction. Many but not all cycloadditions are concerted and thus pericyclic. Nonconcerted cycloadditions are not pericyclic. As a class of addition reaction, cycloadditions permit carbon–carbon bond formation without the use of a nucleophile or electrophile. Cycloadditions can be described using two systems of notation. An older but still common notation is based on the size of linear arrangements of atoms in the reactants. It uses parentheses: where the variables are the numbers of linear atoms in each reactant. The product is a cycle of size . In this system, the standard Diels-Alder reaction is a (4 + 2)-cycloaddition, the 1,3-dipolar cycloaddition is a (3 + 2)-cycloadditi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azide Photolysis Oxygen Trapping
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant application of azides is as a propellant in air bags. Preparation Sodium azide is made industrially by the reaction of nitrous oxide, with sodium amide in liquid ammonia as solvent: : Many inorganic azides can be prepared directly or indirectly from sodium azide. For example, lead azide, used in detonators, may be prepared from the metathesis reaction between lead nitrate and sodium azide. An alternative route is direct reaction of the metal with silver azide dissolved in liquid ammonia. Some azides are produced by treating the carbonate salts with hydrazoic acid. Bonding Azide is isoelectronic with carbon dioxide , cyanate , nitrous oxide , nitronium ion and cyanogen fluoride NCF. Per valence bond theory, azide can be described by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photodissociation
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. Photodissociation is not limited to visible light. Any photon with sufficient energy can affect the chemical bonds of a chemical compound. Since a photon's energy is inversely proportional to its wavelength, electromagnetic radiations with the energy of visible light or higher, such as ultraviolet light, x-rays, and gamma rays can induce such reactions. Photolysis in photosynthesis Photolysis is part of the light-dependent reaction or light phase or photochemical phase or Hill reaction of photosynthesis. The general reaction of photosynthetic photolysis can be given in terms of photons as: :\ce + 2 \text \longrightarrow \ce The chemical nature of "A" depends on the type of organism. Purple sulfur bacteria oxidize hydrogen sulfide () ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
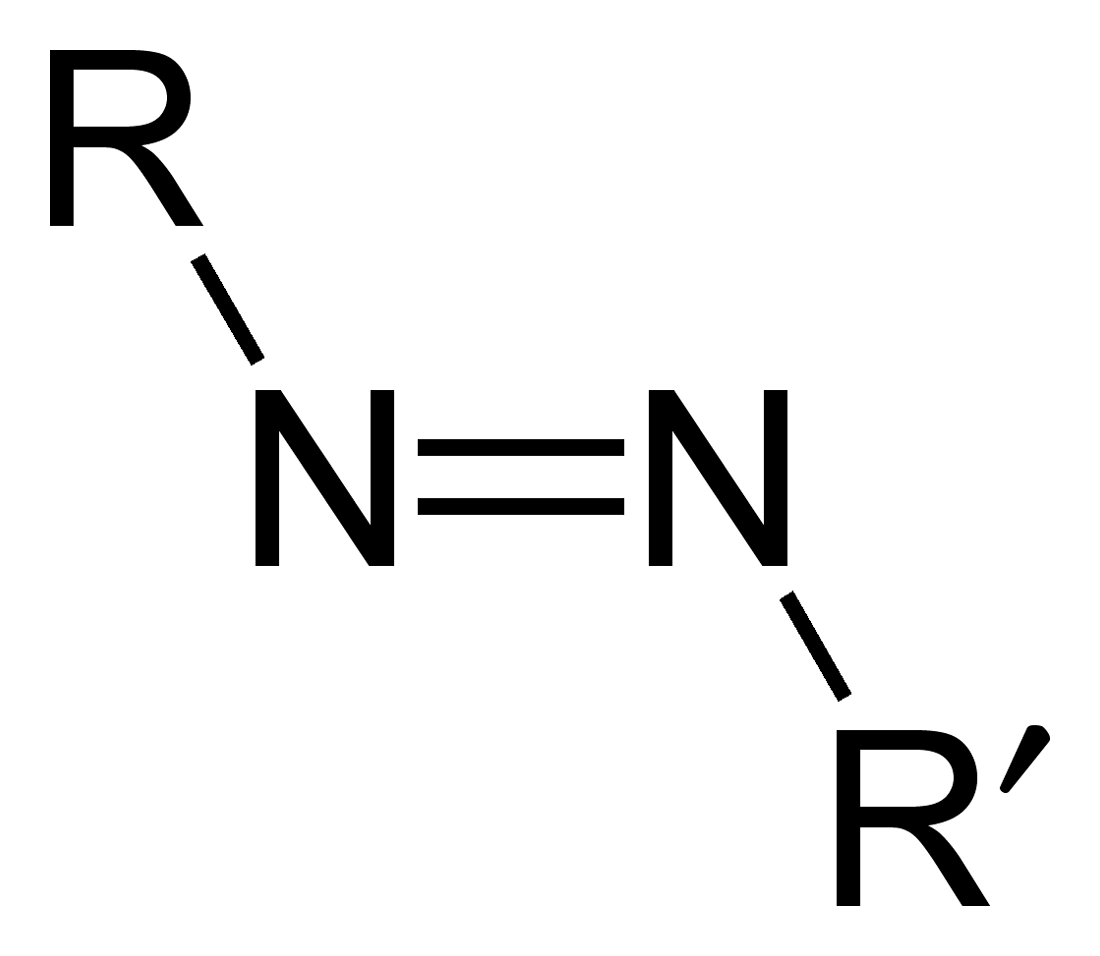
Azo Compound
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene." The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the ''trans'' isomer, but upon illumination, converts to the ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic substitution reaction where an aryl diazonium cation is attacked by another aryl ring, especially those substituted with electron-donating groups: :ArN2+ + Ar'H -> ArN=NAr' + H+ Since diazoniu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diradical
In chemistry, a diradical is a molecular species with two electrons occupying molecular orbitals (MOs) which are degenerate. The term "diradical" is mainly used to describe organic compounds, where most diradicals are extremely reactive and in fact rarely isolated. Diradicals are even-electron molecules but have one fewer bond than the number permitted by the octet rule. Examples of diradical species can also be found in coordination chemistry, for example among bis(1,2-dithiolene) metal complexes. Spin states Diradicals are usually triplets. The phrases ''singlet'' and ''triplet'' are derived from the multiplicity of states of diradicals in electron spin resonance: a singlet diradical has one state (S = 0, Ms = 2*0+1 = 1, ms = 0) and exhibits no signal in EPR and a triplet diradical has 3 states (S = 1, Ms = 2*1+1 = 3, ms = -1; 0; 1) and shows in EPR 2 peaks (if no hyperfine splitting). The triplet state has total spin quantum number S = 1 and is paramagnetic. Therefore, di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homolysis (chemistry)
In chemistry, homolysis () or homolytic fission is the dissociation of a molecular bond by a process where each of the fragments (an atom or molecule) retains one of the originally bonded electrons. During homolytic fission of a neutral molecule with an even number of electrons, two free radicals will be generated. That is, the two electrons involved in the original bond are distributed between the two fragment species. Bond cleavage is also possible by a process called heterolysis. The energy involved in this process is called bond dissociation energy (BDE). BDE is defined as the "enthalpy (per mole) required to break a given bond of some specific molecular entity by homolysis," symbolized as ''D''. BDE is dependent on the strength of the bond, which is determined by factors relating to the stability of the resulting radical species. Because of the relatively high energy required to break bonds in this manner, homolysis occurs primarily under certain circumstances: * Light ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical (chemistry)
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes. A notable example of a radical is the hydroxyl radical (HO·), a molecule that has one unpaired electron on the oxygen atom. Two other examples are triplet oxygen and triplet carbene (꞉) which have two unpaired electrons. Radicals may be generated in a number of ways, but typical methods involve redox reactions. Ionizing radiation, heat, electrical discharges, and electrolysis are known to produce radicals. Radicals are intermediates in many chemical reactions, more so than is apparent from the balanced equations. Radicals are important in combustion, atmospheric chemistry, polymerization, plasma chemistry, biochemistry, and many other chemical processes. A majority of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |