|
Phosphine Imide
In chemistry a phosphine imide (sometimes abbreviated to phosphinimide) also known as a iminophosphorane is a functional group with the formula R3P=NR. While structurally related to phosphine oxide its chemistry has more in common with phosphonium ylides. Anions of this group, with the structure R3P=N−, are called phosphinoimidates and are used as ligands to form phosphinimide complexes which are highly active catalysts in some olefin polymerization reactions. Synthesis Phosphine imides can be isolated as intermediates in the Staudinger reaction and have also been prepared by the action of hydroxylamine-O-sulfonic acid on phosphines, proceeding via a p-aminophosphonium salt. Reactions and applications The functional group will readily hydrolyse to give a phosphine oxide and an amine :R3P=NR' + H2O → R3P=O + R'NH2 Phosphinimide ligands of the general formula NPR3− form transition metal phosphinimide complexeses. Some of these complexes are potential catalysts ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ph3P=NPh
Triphenylphosphine phenylimide is the organophosphorus compound with the formula Ph3P=NPh ( Ph = C6H5). It is a white solid that is soluble in organic solvents. The compound is a prototype of a large class of Staudinger reagents, resulting from the Staudinger reaction. The phosphine imides were first prepared in the laboratory of Nobelist Hermann Staudinger. His synthesis involved the direct reaction of triphenylphosphine with phenylazide. :Ph3P + N3Ph → Ph3P=NPh + N2 X-ray crystallography X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ... establishes that the P-N-C angle is bent (130.4°) and the P-N distance is 160 pm.{{cite journal, title=Die Kristallstrukturen von Ph3PNPh, h3PN(H)PhAuI2], und von 2,3-Bis(triphenylphosphoranimino)maleinsäure-N-methylimid (The Cry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. Fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphine Oxide
Phosphine oxides are phosphorus compounds with the formula OPX3. When X = alkyl or aryl, these are organophosphine oxides. Triphenylphosphine oxide is an example. An inorganic phosphine oxide is phosphoryl chloride (POCl3). Structure and bonding Tertiary phosphine oxides Tertiary phosphine oxides are the most commonly encountered phosphine oxides. With the formula R3PO, they are tetrahedral compounds. They are usually prepared by oxidation of tertiary phosphines. The P-O bond is short and polar. According to molecular orbital theory, the short P–O bond is attributed to the donation of the lone pair electrons from oxygen p-orbitals to the antibonding phosphorus-carbon bonds. The nature of the P–O bond was once hotly debated. Some discussions invoked a role for phosphorus-centered d-orbitals in bonding, but this analysis is not supported by computational analyses. In terms of simple Lewis structure, the bond is more accurately represented as a dative bond, as is currently us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
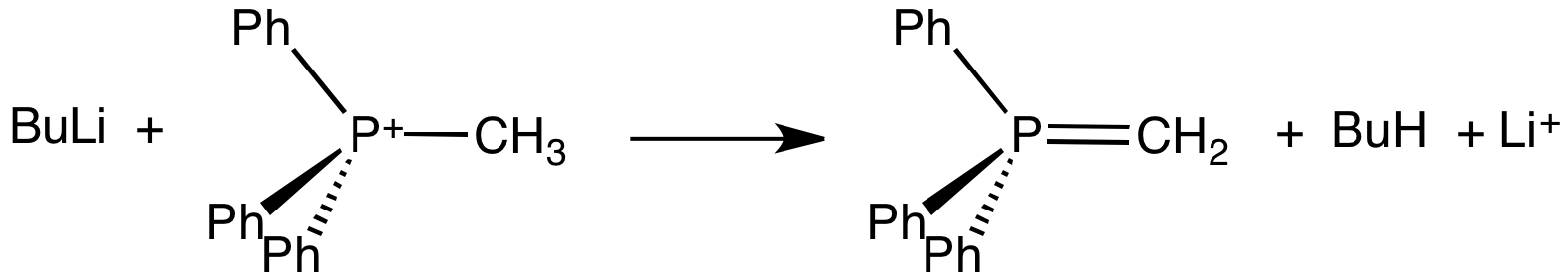
Ylide
An ylide or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. The result can be viewed as a structure in which two adjacent atoms are connected by both a covalent and an ionic bond; normally written X+–Y−. Ylides are thus 1,2-dipolar compounds, and a subclass of zwitterions. They appear in organic chemistry as reagents or reactive intermediates. The class name "ylide" for the compound should not be confused with the suffix "-ylide". Resonance structures Many ylides may be depicted by a multiple bond form in a resonance structure, known as the ylene form, while the actual structure lies in between both forms: : The actual bonding picture of these types of ylides is strictly zwitterionic (the structure on the right) with the strong Coulombic attraction between the " ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphinoimidate
Phosphinoimidates, also known as phophinimides, are the anions derived from phosphine imides with the structure 3P=Nsup>− (R = alkyl or aryl). Phosphinimide ligands are used to for transition metal complexes that are highly active catalysts in some olefin polymerization reactions.Dehnicke, K.; Krieger, M.; Massa, W. Phosphoraneiminato complexes of transition metals. Coord. Chem. Rev. 1999, vol. 182, pp. 19-65. Synthesis of phosphinoimidate complexes Although phosphinoimidates are formally anions, salts of the anions are typically unavailable owing to their high basicity. Instead the ligand is installed indirectly. Staudinger reaction Preparation of phosphinoimidate complexes are achieved in high yield from the corresponding silyl derivatives R3PNSiMe3 (Me = -CH3). These derivatives are prepared by oxidation of phosphine ligands with trimethylsilyl azide, also known as the Staudinger reaction: :PR3 + Me3SiN3 → Me3SiN=PR3 + N2 Starting from these silyl compounds, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphinimide Ligands
Phosphinimide ligands, also known as phosphorane iminato ligands, are any of a class of organic compounds of the general formula NPR3−. The R groups represent organic substituents or, in rare cases, halides or NR2 groups. NPR3− is isoelectronic with phosphine oxides (OPR3) and siloxides ( SiR3sup>−), but far more basic. By varying the R groups on P, a variety of ligands with different electronic and steric properties can be produced, and due to the high oxidation state of phosphorus, these ligands have good thermal stability. Many transition metal phosphinimide complexes have been well-developed as have main group phosphinimide complexes. In main group phosphinimide complexes, only terminal and μ2-N-bridging bonding modes are observed. The terminally bound bent ligands are primarily commonly have M-N-P bond angles ranging from 120-150°. Both the M-N and N-P bond lengths are appropriate for double bonds. This bonding can best be described by a covalent single bond wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Staudinger Reaction
The Staudinger reaction is a chemical reaction of an organic azide with a phosphine or phosphite produces an iminophosphorane. The reaction was discovered by and named after Hermann Staudinger. The reaction follows this stoichiometry: :R3P + R'N3 → R3P=NR' + N2 Staudinger reduction The Staudinger reduction is conducted in two steps. First phosphine imine-forming reaction is conducted involving treatment of the azide with the phosphine. The intermediate, e.g. triphenylphosphine phenylimide, is then subjected to hydrolysis to produce a phosphine oxide and an amine: :R3P=NR' + H2O → R3P=O + R'NH2 The overall conversion is a mild method of reducing an azide to an amine. Triphenylphosphine or tributylphosphine are most commonly used, yielding tributylphosphine oxide or triphenylphosphine oxide as a side product in addition to the desired amine. An example of a Staudinger reduction is the organic synthesis of the pinwheel compound 1,3,5-tris(aminomethyl)-2,4,6-triethylbenz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxylamine-O-sulfonic Acid
Hydroxylamine-''O''-sulfonic acid (HOSA) is the inorganic compound with molecular formula H3NO4S that is formed by the sulfonation of hydroxylamine with oleum. It is a white, water-soluble and hygroscopic, solid, commonly represented by the condensed structural formula H2NOSO3H, though it actually exists as a zwitterion and thus is more accurately represented as +H3NOSO3−. It is used as a reagent for the introduction of amine groups (–NH2), for the conversion of aldehydes into nitriles and alicyclic compound, alicyclic ketones into lactams (cyclic amides), and for the synthesis of variety of nitrogen-containing heterocycles. Preparation According to a laboratory procedure hydroxylamine-''O''-sulfonic acid can be prepared by treating hydroxylamine sulfate with fuming sulfuric acid (oleum). The industrial process is similar. :(NH3OH)2SO4 + 2SO3 → 2H2NOSO3H + H2SO4 The sulfonation of hydroxylamine can also be effected with chlorosulfonic acid by a method first pub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting fish, due to the presence of substituted phosphine and diphosphane (). With traces of present, is spontaneously flammable in air ( pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure. Phosphines are compounds that include and the organophosphines, which are derived from by substituting one or more hydrogen atoms with organic groups. They have the general formula . Phosphanes are saturated phosphorus hydrides of the form , such as triphosphane. Phosphine, PH3, is the smallest of the phosphines and the smallest of the phosphanes. History Philippe Gengembre (1764–1838), a student of Lavois ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal Phosphinimide Complexes
Transition metal phosphinimide complexes are metal complexes that contain phosphinimide ligands of the general formula NPR3− (R = organic substituent). Several coordination modes have been observed, including terminal and various bridging geometries. In the terminal bonding mode the M-N=P core is usually linear but some are quite bent. The preferred coordination type varies with the oxidation state and coligands on the metal and the steric and electronic properties of the R groups on phosphorus. Many transition metal phosphinimide complexes have been well-developed and, more recently, main group phosphinimide complexes have been synthesized. Complexes of Ti, Zr, V, Ta Complexes of Phosphinimide are generally prepared by two routes. For highly electrophilic metal chlorides, the silyl derivative is convenient since is generates volatile trimethylsilyl chloride: :R3PNSiMe3 + LnMCl → R3PN-MLn + ClSiMe3 CpTi(NPR3)Cl2 is prepared by this route. More common are salt-elimination r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |