|
Phenylpiperidine
Phenylpiperidines are chemical compounds with a phenyl moiety directly attached to piperidine. There are a variety of pharmacological effects associated with phenylpiperidines including morphine-like activity or other central nervous system effects. Methylphenidate is a ''benzyl In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group () group. Nomenclature In IUPAC nomenclature, the prefix benzyl refers to a substi ...''piperidine. References Piperidines {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
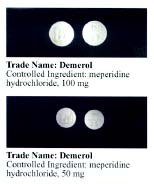
Pethidine
Pethidine, also known as meperidine and sold under the brand name Demerol among others, is a synthetic opioid analgesic, pain medication of the phenylpiperidine class. Synthesized in 1938 as a potential anticholinergic agent by the German chemist Otto Eisleb, its analgesic properties were first recognized by Otto Schaumann while working for IG Farben, Germany. Pethidine is the prototype of a large family of analgesics including the pethidine 4-phenylpiperidines (piminodine, anileridine and others), the prodines (alphaprodine, MPPP, ''etc.''), bemidones (ketobemidone, etc.) and others more distant, including diphenoxylate and analogues. Pethidine is indicated for the treatment of moderate to severe pain, and is delivered as a hydrochloride salt in tablets, as a syrup, or by intramuscular, Subcutaneous injection, subcutaneous, or intravenous injection. For much of the 20th century, pethidine was the opioid of choice for many physicians; in 1975, 60% of doctors prescribed it for acu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Budipine
Budipine (brand name Parkinsan) is an antiparkinson agent marketed for the treatment of Parkinson's disease. While its exact mechanism of action is not well characterized, it is believed to be an NMDA receptor antagonist, but also promoting the synthesis of dopamine. Because it provides additional benefits relative to existing treatments, it probably does not precisely mimic the mechanism of an existing known treatment. Synthesis 4-Phenyl-1-t-butyl-4-piperidinol, (1) 1-t-butyl-3-benzoyl-4-phenyl-4-piperidinol 1831-81-4(3) See also * AD-1211 * Delucemine * Diphenidine * Ephenidine * Fluorolintane * Lanicemine * Methoxphenidine (MXP) * MT-45 * Remacemide Remacemide is a drug which acts as a low-affinity NMDA antagonist with sodium channel blocking properties. It has been studied for the treatment of acute ischemic stroke, epilepsy, Huntington's disease, and Parkinson's disease. Because remacem ... References Antiparkinsonian agents Drugs with unknown mechan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
(+)-CPCA
(+)-CPCA (nocaine, 3α-carbomethoxy-4β-(4-chlorophenyl)-''N''-methylpiperidine aka CTDP 31,446 ) is a stimulant drug similar in structure to pethidine (an opioid that possesses NDRI actions) and to RTI-31, but nocaine is lacking the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine. Since this time many substituted phenylpiperidine derivatives have been discovered, hybridizing the basic nocaine structure with that of other similar molecules such as methylphenidate, meperidine and modafinil to create a large family of derivatives with a range of activity profiles and potential applications. This is a significant field of research with much work ongoing, and dozens of novel compounds have been developed although none have yet come to market. The nocaine family includes a diverse assortment of piperidine based cocaine mimics. The parent compound nocaine was developed in an attempt to develop a substitute drug for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MPPP
Desmethylprodine or 1-methyl-4-phenyl-4-propionoxypiperidine (MPPP, Ro 2-0718) is an opioid analgesic drug developed in the 1940s by researchers at Hoffmann-La Roche. Desmethylprodine has been labeled by the DEA as a Schedule I drug in the United States. It is an analog of pethidine (meperidine) a Schedule II drug. Chemically, it is a reversed ester of pethidine which has about 70% of the potency of morphine. Unlike its derivative prodine, it was not reported to exhibit optical isomerism. It was reported to have 30 times the activity of pethidine and a greater analgesic effect than morphine in rats, and it was demonstrated to cause central nervous system stimulation in mice. History Desmethylprodine was first synthesized in 1947 at Hoffman-LaRoche Laboratories by Albert Ziering and John Lee. They found that it produced effects similar to morphine when administered to rats. Ziering had been searching for synthetic painkillers that were less addictive than morphine. The new ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. Phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring. Nomenclature Usually, a "phenyl group" is synonymous with C6H5− and is represented by the symbol Ph or, archaically, Φ. Benzene is sometimes denoted as PhH. Phenyl groups are generally attached to other atoms or groups. For example, triphenylmethane (Ph3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as a placeholder for the aryl group in chemical structure diagrams, analogous to “R” used for any organic substituent. “Ar” is not to be confused with the elemental symbol for argon. A simple aryl group is phenyl (), a group derived from benzene. Examples of other aryl groups consist of: * The tolyl group () which is derived from toluene (methylbenzene) * The xylyl group (), which is derived from xylene (dimethylbenzene) * The naphthyl group (), which is derived from naphthalene Arylation is the process in which an aryl group is attached to a substituent. It is typically achieved by cross-coupling reactions. Nomenclature The most basic aryl group is phenyl, which is made up of a benzene ring with one hydrogen atom substituted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylphenidate
Methylphenidate, sold under the brand names Ritalin and Concerta among others, is the most widely prescribed central nervous system (CNS) stimulant medication used to treat attention deficit hyperactivity disorder (ADHD) and, to a lesser extent, narcolepsy. It is a primary medication for ADHD; it may be taken by mouth or applied to the skin, and different formulations have varying durations of effect, commonly ranging from 2–4 hours. Though there is little to no evidence, and in some cases contradictory evidence, to support its use as an athletic performance enhancer, cognitive enhancer, aphrodisiac or euphoriant, claims persist that it can be used for these purposes. Common adverse reactions of methylphenidate include: tachycardia, palpitations, headache, insomnia, anxiety, hyperhidrosis, weight loss, decreased appetite, dry mouth, nausea, and abdominal pain. Withdrawal symptoms may include: chills, depression, drowsiness, dysphoria, exhaustion, headaches, irritabili ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reuptake Inhibitor
Reuptake is the reabsorption of a neurotransmitter by a neurotransmitter transporter located along the plasma membrane of an axon terminal (i.e., the pre-synaptic neuron at a synapse) or glial cell after it has performed its function of transmitting a neural impulse. Reuptake is necessary for normal synaptic physiology because it allows for the recycling of neurotransmitters and regulates the level of neurotransmitter present in the synapse, thereby controlling how long a signal resulting from neurotransmitter release lasts. Because neurotransmitters are too large and hydrophilic to diffuse through the membrane, specific transport proteins are necessary for the reabsorption of neurotransmitters. Much research, both biochemical and structural, has been performed to obtain clues about the mechanism of reuptake. Protein structure The first primary sequence of a reuptake protein was published in 1990. The technique for protein sequence determination relied upon the purification, se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine
Monoamine neurotransmitters are neurotransmitters and neuromodulators that contain one amino group connected to an aromatic ring by a two-carbon chain (such as -CH2-CH2-). Examples are dopamine, norepinephrine and serotonin. All monoamines are derived from aromatic amino acids like phenylalanine, tyrosine, and tryptophan by the action of aromatic amino acid decarboxylase enzymes. They are deactivated in the body by the enzymes known as monoamine oxidases which clip off the amine group. Monoaminergic systems, i.e., the networks of neurons that use monoamine neurotransmitters, are involved in the regulation of processes such as emotion, arousal, and certain types of memory. It has also been found that monoamine neurotransmitters play an important role in the secretion and production of neurotrophin-3 by astrocytes, a chemical which maintains neuron integrity and provides neurons with trophic support. Drugs used to increase or reduce the effect of monoamine neurotransmitters are u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Femoxetine
Femoxetine (INN; tentative brand name Malexil; developmental code name FG-4963) is a drug related to paroxetine that was being developed as an antidepressant by Danish pharmaceutical company Ferrosan in 1975 before acquisition of the company by Novo Nordisk. It acts as a selective serotonin reuptake inhibitor (SSRI). Development was halted to focus attention on paroxetine instead, as femoxetine could not be administered as a daily pill. Both femoxetine and paroxetine were invented in the 1970s. Jørgen Anders Christensen's name is on the patents and Jorgen Buus-Lassen's name is on the pharmacology paper. After Ferrosan's acquisition, femoxetine died from neglect. In a separate patent, Ferrosan stated that Femoxetine could be used as an appetite suppressant, using ten times the dosage than for paroxetine, 300 - 400mg daily. Femoxetine has the same stereochemical properties as Nocaine, another agent with a similar structure claimed to have been synthesized using arecoline as t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Norepinephrine Transporter
The norepinephrine transporter (NET), also known as noradrenaline transporter (NAT), is a protein that in humans is encoded by the solute carrier family 6 member 2 (SLC6A2) gene. NET is a monoamine transporter and is responsible for the sodium-chloride (Na+/Cl−)-dependent reuptake of extracellular norepinephrine (NE), which is also known as noradrenaline. NET can also reuptake extracellular dopamine (DA). The reuptake of these two neurotransmitters is essential in regulating concentrations in the synaptic cleft. NETs, along with the other monoamine transporters, are the targets of many antidepressants and recreational drugs. In addition, an overabundance of NET is associated with ADHD. There is evidence that single-nucleotide polymorphisms in the NET gene (''SLC6A2'') may be an underlying factor in some of these disorders. Gene The norepinephrine transporter gene, SLC6A2 is located on human chromosome 16 locus 16q12.2. This gene is encoded by 14 exons. Based on the nucleotide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selective Serotonin Reuptake Inhibitor
Selective serotonin reuptake inhibitors (SSRIs) are a class of drugs that are typically used as antidepressants in the treatment of major depressive disorder, anxiety disorders, and other psychological conditions. SSRIs increase the extracellular level of the neurotransmitter serotonin by limiting its reabsorption (reuptake) into the presynaptic cell. They have varying degrees of selectivity for the other monoamine transporters, with pure SSRIs having strong affinity for the serotonin transporter and only weak affinity for the norepinephrine and dopamine transporters. SSRIs are the most widely prescribed antidepressants in many countries. The efficacy of SSRIs in mild or moderate cases of depression has been disputed and may or may not be outweighed by side effects, especially in adolescent populations. Medical uses The main indication for SSRIs is major depressive disorder; however, they are frequently prescribed for anxiety disorders, such as social anxiety disorder, ge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |