|
Pyrethroid Resistance
A pyrethroid is an organic compound similar to the natural pyrethrins, which are produced by the flowers of pyrethrums ('' Chrysanthemum cinerariaefolium'' and '' C. coccineum''). Pyrethroids are used as commercial and household insecticides. In household concentrations pyrethroids are generally harmless to humans. However, pyrethroids are toxic to insects such as bees, dragonflies, mayflies, gadflies, and some other invertebrates, including those that constitute the base of aquatic and terrestrial food webs. Pyrethroids are toxic to aquatic organisms, especially fish.Pyrethroids fact sheet from the Illinois Department of Public Health. They have been shown to be an effective control measure for malaria outbreaks, through indoor applications. Mode of action P ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allethrin 2D
The allethrins are a group of related synthetic compounds used in insecticides. They are classified as pyrethroids, i.e. synthetic versions of pyrethrin, a chemical with insecticidal properties found naturally in ''Chrysanthemum'' flowers. They were first synthesized in the United States by Milton S. Schechter in 1949. Allethrin was the first pyrethroid. They are commonly used in ultra-low volume sprays for outdoor mosquito control, and in many household insecticides such as RAID, as well as mosquito coils. Chemical structure Allethrin I and allethrin II differ by having a methyl group and a methyl ester, respectively, on one terminus. Each of these allethrins consists of the eight possible stereoisomers. A partly enantiopure variant of allethrin I, consisting of only two stereoisomers in an approximate ratio of 1:1, is called bioallethrin. The same mixture of isomers, but in an approximate ratio of 3:1, is known as esbiothrin. Toxicity Chronic exposure to allethrins alters th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Protein
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane and can either penetrate the membrane ( transmembrane) or associate with one or the other side of a membrane ( integral monotopic). Peripheral membrane proteins are transiently associated with the cell membrane. Membrane proteins are common, and medically important—about a third of all human proteins are membrane proteins, and these are targets for more than half of all drugs. Nonetheless, compared to other classes of proteins, determining membrane protein structures remains a challenge in large part due to the difficulty in establishing experimental conditions that can preserve the correct conformation of the protein in isolation from its native environment. Function Membrane proteins perform a variety of functions vital to the su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
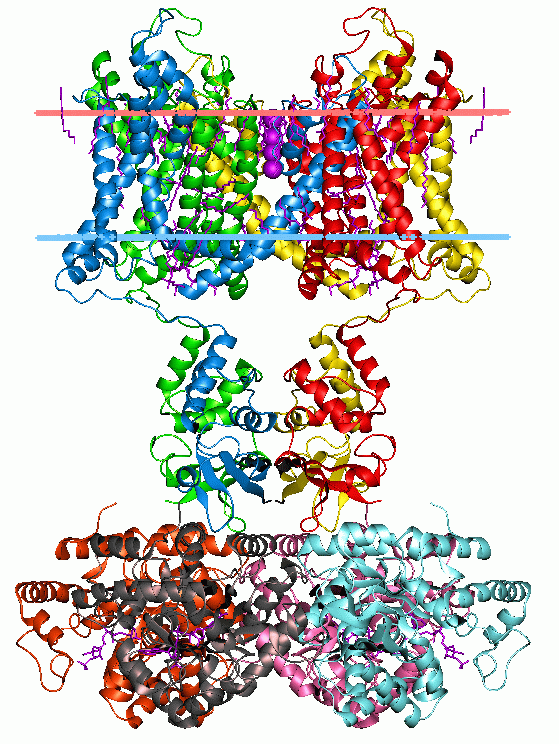
Voltage-gated Ion Channel
Voltage-gated ion channels are a class of transmembrane proteins that form ion channels that are activated by changes in the electrical membrane potential near the channel. The membrane potential alters the conformation of the channel proteins, regulating their opening and closing. Cell membranes are generally impermeable to ions, thus they must diffuse through the membrane through transmembrane protein channels. They have a crucial role in excitable cells such as neuronal and muscle tissues, allowing a rapid and co-ordinated depolarization in response to triggering voltage change. Found along the axon and at the synapse, voltage-gated ion channels directionally propagate electrical signals. Voltage-gated ion-channels are usually ion-specific, and channels specific to sodium (Na+), Voltage-gated potassium channel, potassium (K+), Voltage-dependent calcium channel, calcium (Ca2+), and Chloride channel#General functions, chloride (Cl−) ions have been identified. The opening and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Voltage-gated Calcium Channel
Voltage-gated calcium channels (VGCCs), also known as voltage-dependent calcium channels (VDCCs), are a group of voltage-gated ion channels found in the membrane of excitable cells (''e.g.'', muscle, glial cells, neurons, etc.) with a permeability to the calcium ion Ca2+. These channels are slightly permeable to sodium ions, so they are also called Ca2+-Na+ channels, but their permeability to calcium is about 1000-fold greater than to sodium under normal physiological conditions. At physiologic or resting membrane potential, VGCCs are normally closed. They are activated (''i.e.'': opened) at depolarized membrane potentials and this is the source of the "voltage-gated" epithet. The concentration of calcium (Ca2+ ions) is normally several thousand times higher outside the cell than inside. Activation of particular VGCCs allows a Ca2+ influx into the cell, which, depending on the cell type, results in activation of calcium-sensitive potassium channels, muscular contraction, excit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neuroendocrine Cell
Neuroendocrine cells are cells that receive neuronal input (through neurotransmitters released by nerve cells or neurosecretory cells) and, as a consequence of this input, release messenger molecules ( hormones) into the blood. In this way they bring about an integration between the nervous system and the endocrine system, a process known as neuroendocrine integration. An example of a neuroendocrine cell is a cell of the adrenal medulla (innermost part of the adrenal gland), which releases adrenaline to the blood. The adrenal medullary cells are controlled by the sympathetic division of the autonomic nervous system. These cells are modified postganglionic neurons. Autonomic nerve fibers lead directly to them from the central nervous system. The adrenal medullary hormones are kept in vesicles much in the same way neurotransmitters are kept in neuronal vesicles. Hormonal effects can last up to ten times longer than those of neurotransmitters. Sympathetic nerve fiber impulses sti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are Ribozyme, catalytic RNA molecules, called ribozymes. Enzymes' Chemical specificity, specific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome P450
Cytochromes P450 (CYPs) are a superfamily of enzymes containing heme as a cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are important for the clearance of various compounds, as well as for hormone synthesis and breakdown. In 1963, Estabrook, Cooper, and Rosenthal described the role of CYP as a catalyst in steroid hormone synthesis and drug metabolism. In plants, these proteins are important for the biosynthesis of defensive compounds, fatty acids, and hormones. CYP enzymes have been identified in all kingdoms of life: animals, plants, fungi, protists, bacteria, and archaea, as well as in viruses. However, they are not omnipresent; for example, they have not been found in ''Escherichia coli''. , more than 300,000 distinct CYP proteins are known. CYPs are, in general, the terminal oxidase enzymes in electron transfer chains, broadly categorized as P450-containing systems. The term "P450" is derived fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microsome
In cell biology, microsomes are heterogeneous vesicle-like artifacts (~20-200 nm diameter) re-formed from pieces of the endoplasmic reticulum (ER) when eukaryotic cells are broken-up in the laboratory; microsomes are not present in healthy, living cells. Rough (containing ribosomes) and smooth (without ribosomes) microsomes are made from the endoplasmic reticulum through cell disruption. These microsomes have an inside that is exactly the same as the endoplasmic reticulum lumen. Both forms of microsomes can be purified by a process known as equilibrium density centrifugation. Rough and smooth microsomes do differ in their proteins and rough microsomes have shown occurrence of translation and translocation at the same time besides certain exceptions from proteins in yeast. Signal Hypothesis Microsomes play a role in the signal hypothesis. This hypothesis explores in vitro protein translation for a mRNA encoding secretory protein. When microsomes are present, the proteins s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme Inhibitor
An enzyme inhibitor is a molecule that binds to an enzyme and blocks its activity. Enzymes are proteins that speed up chemical reactions necessary for life, in which substrate molecules are converted into products. An enzyme facilitates a specific chemical reaction by binding the substrate to its active site, a specialized area on the enzyme that accelerates the most difficult step of the reaction. An enzyme inhibitor stops ("inhibits") this process, either by binding to the enzyme's active site (thus preventing the substrate itself from binding) or by binding to another site on the enzyme such that the enzyme's catalysis of the reaction is blocked. Enzyme inhibitors may bind reversibly or irreversibly. Irreversible inhibitors form a chemical bond with the enzyme such that the enzyme is inhibited until the chemical bond is broken. By contrast, reversible inhibitors bind non-covalently and may spontaneously leave the enzyme, allowing the enzyme to resume its function. Rev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperonyl Butoxide
Piperonyl butoxide (PBO) is a pale yellow to light brown liquidNational Toxicology Program, Institute of Environmental Health Sciences, National Institutes of Health (NTP). 1992. National Toxicology Program Chemical Repository Database. Research Triangle Park, North Carolina. organic compound used as a synergist component of pesticide formulations. That is, despite having no pesticidal activity of its own, it enhances the potency of certain pesticides such as carbamates, pyrethrins, pyrethroids, and rotenone. It is a semisynthetic derivative of safrole.Robert L. Metcalf "Insect Control" in Ullmann’s Encyclopedia of Industrial Chemistry" Wiley-VCH, Weinheim, 2002. History PBO was developed in the late 1930s and early 1940s to enhance the performance of the naturally derived insecticide pyrethrum. Pyrethrum is and was an important insecticide against mosquitoes and other disease-carrying vectors, thereby providing public health benefits, e.g., preventing malaria. Although ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Depolarization
In biology, depolarization or hypopolarization is a change within a cell, during which the cell undergoes a shift in electric charge distribution, resulting in less negative charge inside the cell compared to the outside. Depolarization is essential to the function of many cells, communication between cells, and the overall physiology of an organism. Most cells in higher organisms maintain an internal environment that is negatively charged relative to the cell's exterior. This difference in charge is called the cell's membrane potential. In the process of depolarization, the negative internal charge of the cell temporarily becomes more positive (less negative). This shift from a negative to a more positive membrane potential occurs during several processes, including an action potential. During an action potential, the depolarization is so large that the potential difference across the cell membrane briefly reverses polarity, with the inside of the cell becoming positively char ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Repolarization
In neuroscience, repolarization refers to the change in membrane potential that returns it to a negative value just after the depolarization phase of an action potential which has changed the membrane potential to a positive value. The repolarization phase usually returns the membrane potential back to the resting membrane potential. The efflux of potassium (K+) ions results in the falling phase of an action potential. The ions pass through the selectivity filter of the K+ channel pore. Repolarization typically results from the movement of positively charged K+ ions out of the cell. The repolarization phase of an action potential initially results in hyperpolarization, attainment of a membrane potential, termed the afterhyperpolarization, that is more negative than the resting potential. Repolarization usually takes several milliseconds. Repolarization is a stage of an action potential in which the cell experiences a decrease of voltage due to the efflux of potassium (K+) ions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)




