|
Propiophenone
Propiophenone (shorthand: benzoylethane or BzEt) is an aryl ketone. It is a colorless, sweet-smelling liquid that is insoluble in water, but miscible with organic solvents. It is used in the preparation of other compounds. Production Propiophenone can be prepared by Friedel–Crafts reaction of propanoyl chloride and benzene. It is also prepared commercially by ketonization of benzoic acid and propionic acid over calcium acetate Calcium acetate is a chemical compound which is a calcium salt of acetic acid. It has the formula Ca(C2H3O2)2. Its standard name is calcium acetate, while calcium ethanoate is the systematic name. An older name is acetate of lime. The anhydrous ... and alumina at 450–550 °C: :C6H5CO2H + CH3CH2CO2H → C6H5C(O)CH2CH3 + CO2 + H2O Ludwig Claisen discovered that α-methoxystyrene forms this compound when heated for an hour at 300 °C (65% yield). Uses 120px, left, Phenmetrazine, derived from propiophenone, is an appetite suppressant."> ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetophenone
Acetophenone is the organic compound with the formula C6H5C(O)CH3. It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances. Production Acetophenone is formed as a byproduct of the cumene process, the industrial route for the synthesis of phenol and acetone. In the Hock rearrangement of isopropylbenzene hydroperoxide, migration of a methyl group rather than the phenyl group gives acetophenone and methanol as a result of an alternate rearrangement of the intermediate: :C6H5C(CH3)2O2H -> C6H5C(O)CH3 + CH3OH The cumene process is conducted on such a large scale that even the small amount of acetophenone by-product can be recovered in commercially useful quantities. Acetophenone is also generated from ethylbenzene hydroperoxide. Ethylbenzene hydroperoxide is primarily converted to 1-phenylethanol (α-methylbenzyl alcohol) in the process with a small amount of by-product acetophenone. Acetophenone is recovered or hydrogena ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenmetrazine
Phenmetrazine (INN, USAN, BAN) (brand name Preludin, and many others) is a stimulant drug first synthesized in 1952 and originally used as an appetite suppressant, but withdrawn from the market in the 1980s due to widespread abuse. It was initially replaced by its analogue phendimetrazine (under the brand name Prelu-2) which functions as a prodrug to phenmetrazine, but now it is rarely prescribed, due to concerns of abuse and addiction. Chemically, phenmetrazine is a substituted amphetamine containing a morpholine ring. History Phenmetrazine was first patented in Germany in 1952 by Boehringer-Ingelheim, with some pharmacological data published in 1954. It was the result of a search by Thomä and Wick for an anorectic drug without the side-effects of amphetamine. Phenmetrazine was introduced into clinical use in 1954 in Europe. Medical use In clinical use, phenmetrazine produces less nervousness, hyperexcitability, euphoria and insomnia than drugs of the amphetamine family ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenmetrazine
Phenmetrazine (INN, USAN, BAN) (brand name Preludin, and many others) is a stimulant drug first synthesized in 1952 and originally used as an appetite suppressant, but withdrawn from the market in the 1980s due to widespread abuse. It was initially replaced by its analogue phendimetrazine (under the brand name Prelu-2) which functions as a prodrug to phenmetrazine, but now it is rarely prescribed, due to concerns of abuse and addiction. Chemically, phenmetrazine is a substituted amphetamine containing a morpholine ring. History Phenmetrazine was first patented in Germany in 1952 by Boehringer-Ingelheim, with some pharmacological data published in 1954. It was the result of a search by Thomä and Wick for an anorectic drug without the side-effects of amphetamine. Phenmetrazine was introduced into clinical use in 1954 in Europe. Medical use In clinical use, phenmetrazine produces less nervousness, hyperexcitability, euphoria and insomnia than drugs of the amphetamine family ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha And Beta Carbon
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule. Numeric locants The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of numeric prefixes to indicate the position of substituents, generally by identifying the parent hydrocarbon chain and assigning the carbon atoms based on their substituents in order of precedence. For example, there are at least two isomers of the linear form of pentanone, a ketone that contains a chain of exactly five carbon atoms. There is an oxygen atom bonded to one of the middle three carbons (if it were bonded to an end carbon, the molecule would be an aldehyde, not a ketone), but it is not clear where it is located. In this example, the carbon atoms are numbered from one to five, which starts at one end and proceeds sequentially along the chain. Now the position of the oxygen atom can be defined as on carbon atom number two, three or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ludwig Claisen
Rainer Ludwig Claisen (; 14 January 1851 – 5 January 1930) was a German chemist best known for his work with condensations of carbonyls and sigmatropic rearrangements. He was born in Cologne as the son of a jurist and studied chemistry at the university of Bonn (1869), where he became a member of K.St.V. Arminia. He served in the army as a nurse in 1870–1871 and continued his studies at Göttingen University. He returned to the University of Bonn in 1872 and started his academic career at the same university in 1874. He died in 1930 in Godesberg am Rhein (near Bonn). Career Scientific contributions * Described the condensation of aromatic aldehydes with aliphatic aldehydes or ketones in 1881. This variation of the now well-known aldol condensation reaction is called the Claisen–Schmidt condensation. * Discovered (1887) the condensation reaction of an ester with an activated methylene group, now known as the Claisen condensation. * Synthesis of cinnamates by react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Acetate
Calcium acetate is a chemical compound which is a calcium salt of acetic acid. It has the formula Ca(C2H3O2)2. Its standard name is calcium acetate, while calcium ethanoate is the systematic name. An older name is acetate of lime. The anhydrous form is very hygroscopic; therefore the monohydrate (Ca(CH3COO)2•H2O) is the common form. Production Calcium acetate can be prepared by soaking calcium carbonate (found in eggshells, or in common carbonate rocks such as limestone or marble) or hydrated lime in vinegar: :CaCO3(s) + 2CH3COOH(aq) → Ca(CH3COO)2(aq) + H2O(l) + CO2(g) :Ca(OH)2(s) + 2CH3COOH(aq) → Ca(CH3COO)2(aq) + 2H2O(l) Since both reagents would have been available pre-historically, the chemical would have been observable as crystals then. Uses * In kidney disease, blood levels of phosphate may rise (called hyperphosphatemia) leading to bone problems. Calcium acetate binds phosphate in the diet to lower blood phosphate levels. * Calcium acetate is used as a food addit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
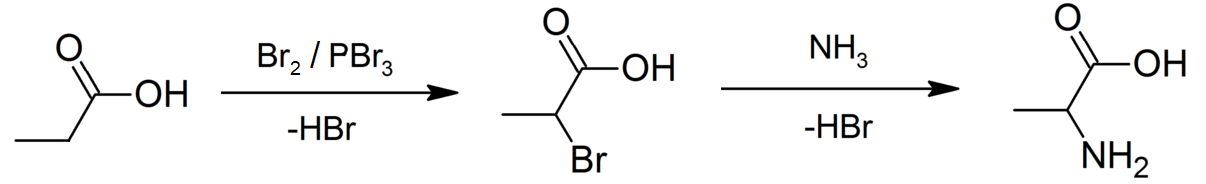
Propionic Acid
Propionic acid (, from the Greek words πρῶτος : ''prōtos'', meaning "first", and πίων : ''píōn'', meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula CH3CH2CO2H. It is a liquid with a pungent and unpleasant smell somewhat resembling body odor. The anion CH3CH2CO2− as well as the salts and esters of propionic acid are known as propionates or propanoates. History Propionic acid was first described in 1844 by Johann Gottlieb, who found it among the degradation products of sugar. Over the next few years, other chemists produced propionic acid by different means, none of them realizing they were producing the same substance. In 1847, French chemist Jean-Baptiste Dumas established all the acids to be the same compound, which he called propionic acid, from the Greek words πρῶτος (prōtos), meaning ''first'', and πίων (piōn), meaning ''fat'', because it is the smallest H(CH2)''n''COOH acid that exhib ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
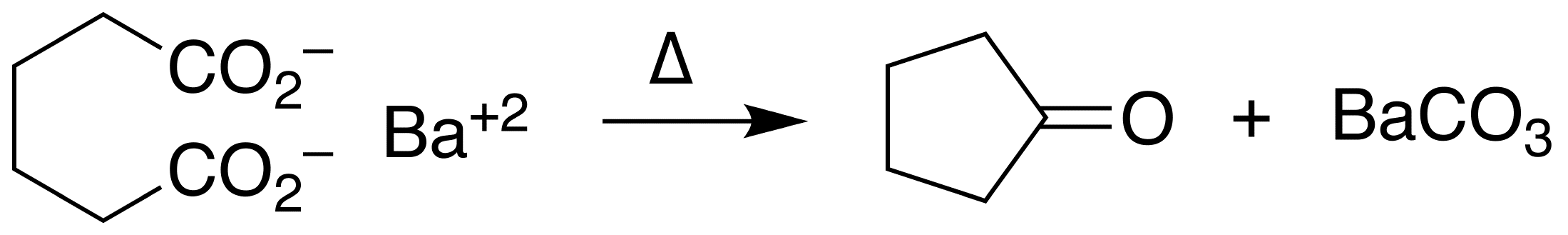
Ketonization
In organic chemistry, ketonic decarboxylation (also known as decarboxylative ketonization) is a type of organic reaction and a decarboxylation converting two equivalents of a carboxylic acid () to a symmetric ketone () by the application of heat with expulsion of one equivalent of water () and one equivalent of carbon dioxide (): :\ce\mathbf + \ce\mathbf \longrightarrow \ce\mathbf + \ce Bases promote this reaction. The reaction mechanism likely involves nucleophilic attack of the alpha-carbon of one acid group on the other acid group's carbonyl (), possibly as a concerted reaction with the decarboxylation. The initial formation of an intermediate carbanion via decarboxylation of one of the acid groups prior to the nucleophilic attack has been proposed, but is unlikely since the byproduct resulting from the carbanion's protonation by the acid has never been reported. This reaction is different from oxidative decarboxylation, which proceeds through a radical mechanism and is charact ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzoic Acid
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, which was for a long time its only source. Benzoic acid occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites. Salts of benzoic acid are used as food preservatives. Benzoic acid is an important precursor for the industrial synthesis of many other organic substances. The salts and esters of benzoic acid are known as benzoates . History Benzoic acid was discovered in the sixteenth century. The dry distillation of gum benzoin was first described by Nostradamus (1556), and then by Alexius Pedemontanus (1560) and Blaise de Vigenère (1596). Justus von Liebig and Friedrich Wöhler determined the composition of benzoic acid. These latter also investigated how hippuric acid is related ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structure, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. History Discovery The word "''benzene''" derives from "''gum benzoin''" (benzoin res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2.png)